Abstract
Purpose
It is well known that CPT-11 (irinotecan) is biotransformed to its active metabolite, SN-38, by carboxylesterase in the liver and other tissues. However, little is known about its pharmacokinetics (PK) when administered intraperitoneally. The aim of our study was to develop a population pharmacokinetic model for CPT-11 and SN-38 following the intraperitoneal (IP) administration of CPT-11.
Methods
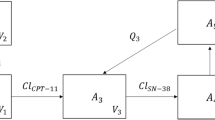
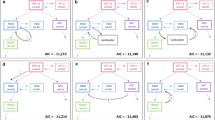
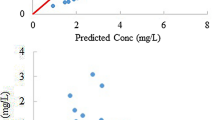
Pharmacokinetic data obtained from 16 gastric adenocarcinoma patients with peritoneal seeding were used. Administered doses ranged from 50 to 250 mg/m2. To measure CPT-11 and SN-38 levels, we collected samples of peritoneal fluid, plasma and urine 0, 0.5, 1.5, 2, 3.5, 8, 12, 25.5, 49 and 56 h after IP infusion. Several multicompartmental pharmacokinetic models were tested for CPT-11 and SN-38 in the sampled peritoneal fluid, plasma and urine. NONMEM ver. 6 was used throughout the model-building process.
Results
Peak concentrations were achieved earlier for peritoneal SN-38 than for plasma SN-38. The apparent metabolic clearance of peritoneal and plasma CPT-11 to peritoneal and plasma SN-38 accounted for 0.2 and 7.3% of the total clearance of peritoneal and plasma CPT-11, respectively. The typical values of steady-state volume of distribution (Vss) (46.6 L/m2), inter-compartment clearance (6.70 L/h/m2) and clearance (16.0 L/h/m2) for plasma CPT-11 were estimated in a two-compartment PK model.
Conclusions
Our results demonstrate that a small fraction of intraperitoneally administered CPT-11 was metabolized in situ to active SN-38 and that the Vss of plasma CPT-11 following IP administration in our patient cohort was lower than that estimated in previous reports following the intravenous administration of CPT-11.





Similar content being viewed by others
References
Chabot GG (1997) Clinical pharmacokinetics of irinotecan. Clin Pharmacokinet 33(4):245–259
Mathijssen RH, van Alphen RJ, Verweij J, Loos WJ, Nooter K, Stoter G, Sparreboom A (2001) Clinical pharmacokinetics and metabolism of irinotecan (CPT-11). Clin Cancer Res 7(8):2182–2194
Stella VH, Borchardt RT, Hageman MJ, Oliyai R, Maag H, Tilley J (eds) (2007) Prodrugs challenges and rewards. Springer/AAPS Press, New York
Sano K, Yoshikawa M, Hayasaka S, Satake K, Ikegami Y, Yoshida H, Ishikawa T, Sawada S, Tanabe S (2003) Simple non-ion-paired high-performance liquid chromatographic method for simultaneous quantitation of carboxylate and lactone forms of 14 new camptothecin derivatives. J Chromatogr B Analyt Technol Biomed Life Sci 795(1):25–34
Burke TG, Mi Z (1994) The structural basis of camptothecin interactions with human serum albumin: impact on drug stability. J Med Chem 37(1):40–46
Choi MK, Ahn BJ, Yim DS, Park YS, Kim S, Sohn TS, Noh JH, Heo JS, Lee J, Park SH, Park JO, Lim HY, Kang WK (2010) Phase I study of intraperitoneal irinotecan in patients with gastric adenocarcinoma with peritoneal seeding. Cancer Chemother Pharmacol. doi:10.1007/s00280-010-1272-6
Sparreboom A, de Jonge MJ, de Bruijn P, Brouwer E, Nooter K, Loos WJ, van Alphen RJ, Mathijssen RH, Stoter G, Verweij J (1998) Irinotecan (CPT-11) metabolism and disposition in cancer patients. Clin Cancer Res 4(11):2747–2754
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO (2009) Prediction corrected visual predictive checks. Available at: http://www.go-acop.org/acop2009/posters
Xie R, Mathijssen RH, Sparreboom A, Verweij J, Karlsson MO (2002) Clinical pharmacokinetics of irinotecan and its metabolites: a population analysis. J Clin Oncol 20(15):3293–3301
Chabot GG, Abigerges D, Catimel G, Culine S, de Forni M, Extra JM, Mahjoubi M, Herait P, Armand JP, Bugat R et al (1995) Population pharmacokinetics and pharmacodynamics of irinotecan (CPT-11) and active metabolite SN-38 during phase I trials. Ann Oncol 6(2):141–151
Catimel G, Chabot GG, Guastalla JP, Dumortier A, Cote C, Engel C, Gouyette A, Mathieu-Boue A, Mahjoubi M, Clavel M (1995) Phase I and pharmacokinetic study of irinotecan (CPT-11) administered daily for three consecutive days every three weeks in patients with advanced solid tumors. Ann Oncol 6(2):133–140
Klein CE, Gupta E, Reid JM, Atherton PJ, Sloan JA, Pitot HC, Ratain MJ, Kastrissios H (2002) Population pharmacokinetic model for irinotecan and two of its metabolites, SN-38 and SN-38 glucuronide. Clin Pharmacol Ther 72(6):638–647
Maruyama M, Toukairin Y, Baba H, Kure N, Nagahama T, Ebuchi M (2001) Pharmacokinetic study of the intraperitoneal administration of CPT-11 for patients with peritoneal seedings of gastric and colonic cancers. Gan To Kagaku Ryoho 28(11):1505–1507
Natsui S, Maruyama M, Ochiai T, Hasegawa K, Takashima I, Nagahama T, Ebuchi M (2002) Pharmacokinetic study of CPT-11, SN-38 and SN-38 glucuronide in the ascites, plasma and bile after intraperitoneal administration of CPT-11. Gan To Kagaku Ryoho 29(12):2188–2190
Guichard S, Chatelut E, Lochon I, Bugat R, Mahjoubi M, Canal P (1998) Comparison of the pharmacokinetics and efficacy of irinotecan after administration by the intravenous versus intraperitoneal route in mice. Cancer Chemother Pharmacol 42(2):165–170
Maruyama M, Nagahama T, Yuasa Y (1999) Intraperitoneal versus intravenous CPT-11 for peritoneal seeding and liver metastasis. Anticancer Res 19(5B):4187–4191
Ragot S, Marquet P, Lachatre F, Rousseau A, Lacassie E, Gaulier JM, Dupuy JL, Lachatre G (1999) Sensitive determination of irinotecan (CPT-11) and its active metabolite SN-38 in human serum using liquid chromatography-electrospray mass spectrometry. J Chromatogr B Biomed Sci Appl 736(1–2):175–184
Khan S, Ahmad A, Ahmad I (2003) A sensitive and rapid liquid chromatography tandem mass spectrometry method for quantitative determination of 7-ethyl-10-hydroxycamptothecin (SN-38) in human plasma containing liposome-based SN-38 (LE-SN38). Biomed Chromatogr 17(8):493–499
Poujol S, Pinguet F, Malosse F, Astre C, Ychou M, Culine S, Bressolle F (2003) Sensitive HPLC-fluorescence method for irinotecan and four major metabolites in human plasma and saliva: application to pharmacokinetic studies. Clin Chem 49(11):1900–1908
Slatter JG, Schaaf LJ, Sams JP, Feenstra KL, Johnson MG, Bombardt PA, Cathcart KS, Verburg MT, Pearson LK, Compton LD, Miller LL, Baker DS, Pesheck CV, Lord RS 3rd (2000) Pharmacokinetics, metabolism, and excretion of irinotecan (CPT-11) following I.V. infusion of [(14)C]CPT-11 in cancer patients. Drug Metab Dispos 28(4):423–433
Sasaki Y, Yoshida Y, Sudoh K, Hakusui H, Fujii H, Ohtsu T, Wakita H, Igarashi T, Itoh K (1995) Pharmacological correlation between total drug concentration and lactones of CPT-11 and SN-38 in patients treated with CPT-11. Jpn J Cancer Res 86(1):111–116
Hamada A, Aoki A, Terazaki H, Ito K, Yokoo K, Sasaki Y, Saito H (2005) Pharmacokinetic changes of irinotecan by intestinal alkalinization in an advanced colorectal cancer patient. Ther Drug Monit 27(4):536–538
Transparency declarations
A part of this manuscript was presented at the American Society for Clinical Pharmacology and Therapeutics 2010 annual meeting held in Atlanta, GA, USA. With regard to the conflict of interest, the authors have nothing to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahn, BJ., Choi, M.K., Park, Y.S. et al. Population pharmacokinetics of CPT-11 (irinotecan) in gastric cancer patients with peritoneal seeding after its intraperitoneal administration. Eur J Clin Pharmacol 66, 1235–1245 (2010). https://doi.org/10.1007/s00228-010-0885-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-010-0885-3