Abstract
Purpose
Diurnal variation can affect drug pharmacokinetics. Fesoterodine is a new antimuscarinic drug for the treatment of overactive bladder (OAB). We estimated the relative bioavailability of 5-hydroxymethyl tolterodine (5-HMT), the active metabolite of fesoterodine, following nighttime and daytime administration.
Methods
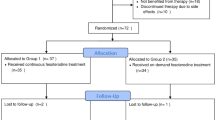
In this randomized, open-label, two–period, two–treatment crossover, single-dose study, healthy subjects received daytime and nighttime oral dosing of fesoterodine 8-mg sustained-release tablets, separated by a minimum 60-h washout period. Blood samples for 5–HMT PK determination were collected before dosing and at specified intervals up to 48 h postdose. Safety was assessed by adverse event (AE) reports.
Results
Fourteen subjects completed the study. Plasma concentration versus time profiles (AUC) of 5-HMT were similar for daytime and nighttime dosing. Mean \( AU{C_\infty } \) 5-HMT values were 47.9 and 51.4 ng h/mL for nighttime and daytime dosing, respectively; the mean time to reach maximum concentration (Cmax) values were 3.9 and 5.0 ng/mL, respectively. Nighttime versus daytime \( AU{C_\infty } \) and Cmax ratios of 5-HMT were 93 and 79%, respectively; 90% confidence intervals (CIs) indicated equivalence for \( AU{C_\infty } \) but not for Cmax. The median time to reach maximum concentration (Tmax) was 5.0 h for both dosing regimens, and the mean terminal elimination half-life (T½) was 5.9 and 5.7 h for nighttime and daytime dosing, respectively. Seven treatment-related AEs, most commonly headache, occurred in five subjects.
Conclusions
The AUC values for daytime and nighttime administration of fesoterodine were equivalent. The 21% reduction in the Cmax for nighttime dosing is unlikely to be clinically relevant. No safety issues were apparent. These results support both daytime and nighttime administration of fesoterodine for OAB treatment.

Similar content being viewed by others
References
Abrams P, Cardozo L, Fall M et al (2003) The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 61(1):37–49
Abrams P, Artibani W, Cardozo L et al (2006) Reviewing the ICS 2002 terminology report: the ongoing debate. Neurourol Urodyn 25:293
Milsom I, Abrams P, Cardozo L et al (2001) How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. Br J Urol Int 87(9):760–766
Stewart WF, Van Rooyen JB, Cundiff GW et al (2003) Prevalence and burden of overactive bladder in the United States. World J Urol 20(6):327–336
Carr LK (2008) Overactive bladder. Can J Urol 15[Suppl 1]:32–36 discussion 36
Chapple C, Van Kerrebroeck P, Tubaro A et al (2007) Clinical efficacy, safety, and tolerability of once-daily fesoterodine in subjects with overactive bladder. Eur Urol 52(4):1204–1212
Nitti V, Dmochowski R, Sand P et al (2007) Efficacy, safety, and tolerability of fesoterodine in subjects with overactive bladder. J Urol 178:2488–2494
Michel MC (2008) Fesoterodine: a novel muscarinic receptor antagonist for the treatment of overactive bladder syndrome. Expert Opin Pharmacother 9(10):1787–1796
Tubaro A, De Nunzio C (2004) Comparison of peripherally active substance for treatment of detrusor overactivity: what is new; what is in the pipeline. EAU Update Ser 2(4):161–169
Cole P (2004) Fesoterodine, an advanced antimuscarinic for the treatment of overactive bladder: a safety update. Drugs Future 29(7):715–720
Malhotra B, Sachse R, Wood N (2009) Influence of food on the pharmacokinetic profile of fesoterodine. Int J Clin Pharmacol Ther 47(6):384–390
Baraldo M (2008) The influence of circadian rhythms on the kinetics of drugs in humans. Expert Opin Drug Metab Toxicol 4(2):175–192
Hatlebakk JG, Katz PO, Kuo B et al (1998) Nocturnal gastric acidity and acid breakthrough on different regimens of omeprazole 40 mg daily. Aliment Pharmacol Ther 12(12):1235–1240
Pehlivanov ND, Olyaee M, Sarosiek I et al (2003) Comparison of morning and evening administration of rabeprazole for gastro-oesophageal reflux and nocturnal gastric acid breakthrough in patients with reflux disease: a double-blind, cross-over study. Aliment Pharmacol Ther 18(9):883–890
Gupta SK, Sathayan G (1999) Pharmacokinetics of an oral once-a-day controlled-release oxybutynin formulation compared with immediate-release oxybutynin. J Clin Pharmacol 39:289–296
Rackley R, Weiss JP, Rovner ES et al (2006) Nighttime dosing with tolterodine reduces overactive bladder-related nocturnal micturitions in patients with overactive bladder and nocturia. Urology 67(4):731–736
Center for Drug Evaluation and Research (2007) Clinical Pharmacology and Biopharmaceutics Review application NDA 22-103. Silver Spring, MD
May K, Westphal K, Giessmann T et al (2008) Disposition and antimuscarinic effects of the urinary bladder spasmolytics propiverine: influence of dosage forms and circadian-time rhythms. J Clin Pharmacol 48(5):570–579
MacDiarmid S, Sandage BW Jr, Malhotra BK (2008) The effects of reformulation: improved therapeutic index. Curr Urol Rep 9(6):465–471
(2003) Guidance for industry: bioavailability and bioequivalence studies for orally administered drug products—general consideration. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Rockville
Olsson B, Szamosi J (2001) Multiple dose pharmacokinetics of a new once daily extended release tolterodine formulation versus immediate release tolterodine. Clin Pharmacokinet 40(3):227–235
Anderson RU, Mobley D, Blank B et al (1999) Once daily controlled versus immediate release oxybutynin chloride for urge urinary incontinence. OROS Oxybutynin Study Group. J Urol 161(6):1809–1812
van Kerrebroeck P, Kreder K, Jonas U et al (2001) Tolterodine once-daily: superior efficacy and tolerability in the treatment of the overactive bladder. Urology 57(3):414–421
Versi E, Appell R, Mobley D et al (2000) Dry mouth with conventional and controlled-release oxybutynin in urinary incontinence. The Ditropan XL Study Group. Obstet Gynecol 95(5):718–721
Junemann KP, Hessdorfer E, Unamba-Oparah I et al (2006) Propiverine hydrochloride immediate and extended release: comparison of efficacy and tolerability in patients with overactive bladder. Urol Int 77(4):334–339
Sathyan G, Chancellor MB, Gupta SK (2001) Effect of OROS controlled-release delivery on the pharmacokinetics and pharmacodynamics of oxybutynin chloride. Br J Clin Pharmacol 52(4):409–417
Sanctura (trospium chloride) (2007) Full prescribing information. Allergan, Irvine
Dmochowski RR, Peters KM, Morrow JD et al (2009) Randomized, double-blind, placebo-controlled study of flexible-dose fesoterodine in subjects with overactive bladder. Urology (in press)
Wyndaele JJ, Goldfischer ER, Morrow JD et al (2009) Effects of flexible-dose fesoterodine on overactive bladder symptoms and treatment satisfaction: an open-label study. Int J Clin Pract 63(4):560–567
Acknowledgments
Editorial assistance was provided by Tiffany Brake, PhD, from Complete Healthcare Communications, Inc., and was funded by Pfizer Inc, who also funded this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
ClinicalStudyResults.org Protocol No. A0221062.
Rights and permissions
About this article
Cite this article
Malhotra, B.K., Crownover, P.H., LaBadie, R. et al. The pharmacokinetic profile of fesoterodine 8 mg with daytime or nighttime dosing. Eur J Clin Pharmacol 66, 171–176 (2010). https://doi.org/10.1007/s00228-009-0748-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-009-0748-y