Abstract
Background and purpose
Many studies have been done to determine the distribution of acetylator phenotypes among populations of different geographic origin. The goal of this study was to investigate the acetylator phenotypes of the Iranian population and compare them between tubercular patients and healthy subjects.
Methods
The study population consisted of two groups; the first group included 100 newly diagnosed tubercular patients and the second group consisted of 100 healthy subjects. Acetylator phenotype was determined from the metabolic ratio of acetyl-isoniazid to isoniazid in the plasma samples. Metabolic ratio was used to classify subjects as slow (≤0.70) or fast acetylators (>0.70).
Results
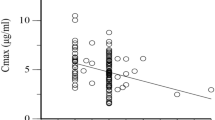
In the tubercular patients, the frequencies of slow and fast acetylator phenotypes were 62 and 38%, respectively. Of the healthy individuals, 45% were found to be slow acetylators and the remaining 55% were fast acetylators.
Conclusion
It seems that tubercular patients metabolize isoniazid more slowly than healthy individuals.


Similar content being viewed by others
References
Schaaf HS, Parkin DP, Seifart HI, Werely CJ, Hesseling PB, van Helden PD, Maritz JS, Donald PR (2005) INH pharmacokinetic in children treated for respiratory tuberculosis. Arch Dis Child 90:614–618
Weber WW, Hein DW (1979) Clinical pharmacokinetics of INH. Clin Pharmacokinet 4:401–422
The American Pharmacists Association (APhA) (2006) Drug information handbook international, 14th edn. Lexi-Comp, The American Pharmacists Association (APhA), Hudson, OH, pp 936–938
Meyer UA, Zanger UM, Skoda RC, Grant D, Blum M (1990) Genetic polymorphism of drug metabolism. Progress in liver diseases. Prog Liver Dis 9:307–323
Evans DA, Manley KA, McKusick VA (1960) Genetic control of INH metabolism in man. Br Med J 2:485–491
Schoeman JF, Morkel A, Seifart HI, Parkin DP, Van Helden PD, Hewlett RH, Donald PR (1998) Massive posterior fossa tuberculous abscess developing in a young child treated for miliary tuberculosis. Possible role of very rapid acetylation of INH. Pediatr Neurosurg 29:64–68
The American Society of Health-System Pharmacists (AHFS). Drug Information 2007:554–559
Possuelo LG, Castelan JA, de Brito TC, Ribeiro AW, Cafrune PI, Picon PD, Santos AR, Teixeira RL, Gregianini TS, Hutz MH, Rossetti ML, Zaha A (2008) Association of slow N-acetyltransferase 2 profile and anti-TB drug-induced hepatotoxicity in patients from Southern Brazil. Eur J Clin Pharmacol 64:673–678
Steichen O, Martinez-Almoyna L, De Broucker T (2006) Isoniazid induced neuropathy: consider prevention. Rev Mal Respir 23:157–160
Meyer UA, Zanger UM (1997) Molecular mechanisms of genetic polymorphisms of drug metabolism. Annu Rev Pharmacol Toxicol 37:269–296
Sunahara S, Uren M, Agawa M (1961) Genetic and geographical studies on INH inactivation. Science 134:1530–1531
Seifart HI, Parkin DP, Botha FJ, Donald PR, Van Der Walt BJ (2001) Population screening for INH acetylator phenotype. Pharmacoepidemiol Drug Saf 10:127–134
World Health Organization Global Tuberculosis Programme (2003) Treatment of tuberculosis: guidelines for national programmes. WHO/CDS/TB/2003.13, 3rd edn. WHO, Geneva
Wilcke JT, Døssing M, Angelo HR, Askgaard D, Rønn A, Christensen HR (2004) Unchanged acetylation of INH by alcohol intake. Int J Tuberc Lung Dis 8:1373–1376
Fox HH, Gibas JT (1953) Synthetic tuberculostat. Acyl derivatives of isonicotinyl hydrazine. J Org Chem 18:1375–1379
Fox HH, Gibas JT (1953) Synthetic tuberculostat. Alkidenyl derivatives of isonicotinyl hydrazine. J Org Chem 18:983–989
Fox HH, Gibas T (1953) Synthetic tuberculostat. Mono-alkyl derivatives of isonicotinyl hydrazine. J Org Chem 18:994–1002
Hutchings A, Monie RD, Spragg B, Routledge PA (1983) High performance liquid chromatographic analysis of INH and acetylINH in biological fluids. J Chromatogr 277:385–390
Hutchings A, Routledge PA (1986) A simple method for determining acetylator phenotype using INH. Br J Clin Pharmacol 22:343–345
Miscoria G, Leneveu A, Walle C, Roux A (1988) Application of a method of analysis using high performance liquid chromatography of INH and acetylINH to determine the phenotype of acetylation. Ann Biol Clin 46:734–740
Kohno H, Kubo H, Takada A, Mori M, Arias TD (1996) INH acetylation phenotyping in the Japanese: the molar metabolic ratio INH/AcINH. Am J Ther 3:74–78
Gupta RC, Nair CR, Jindal SK, Malik SK (1984) Incidence of INH acetylation phenotype in North Indians. Int J Clin Pharmacol Ther Toxicol 22:259–264
Matar KM, Mayet AY, Ayoola EA, Bawazir SA, Al-Faleh FZ, Al-Wazzan A (2004) INH acetylation in Saudi Arabs. J Clin Pharm Ther 29:443–447
Irshaid YM, Al-Hadidi HF, Abuirjeie MA, Rawashdeh NM (1991) N-acetylation phenotyping using dapsone in a Jordanian population. Br J Clin Pharmacol 32:289–293
Sohrani AS, Ahmad B, Janbaz KH (1995) Acetylation percentage method for determination of acetylator status in human volunteers and tuberculosis patients. Pak J Pharma Sci 8:11–16
Kita T, Tanigawara Y, Chikazawa S, Hatanaka H, Sakaeda T, Komada F, Iwakawa S, Okumura K (2001) N-acetyltransferase2 genotype correlated with isoniazid acetylation in Japanese tuberculous patients. Biol Pharm Bull 24:544–549
Hassanzadeh MK, Khashayarmanesh Z (1999) N-acetylation phenotyping using INH in an Iranian and an Afghani population. Indian J Pharm Sci 61:297–300
Bakayev VV, Mohamadi F, Javeri A, Masjedi MR, Velayati AA (2004) Arylamine N-acetyltransferase 2 slow acetylator polymorphisms in unrelated Iranian individuals. Eur J Clin Pharmacol 60:467–471
O'Neil WM, Drobitch RK, MacArthur RD, Farrough MJ, Doll MA, Fretland AJ, Hein DW, Crane LR, Svensson CK (2000) Acetylator phenotype and genotype in patients infected with HIV: discordance between methods for phenotype determination and genotype. Pharmacogenetics 10:171–182
Singh N, Dubey S, Chinnaraj S, Golani A, Maitra A (2009) Study of NAT2 gene polymorphisms in an Indian population: association with plasma isoniazid concentration in a cohort of tuberculosis patients. Mol Diagn Ther 13:49–58
Torkaman-Boutorabi A, Hoormand M, Naghdi N, Bakhshayesh M, Milami I (2007) Genotyping and allele frequencies of N-acetyltransferase 2 and glutathione S-transferase in the Iranian population. Clin Exp Pharmacol Physiol 34:1207–1211
Küpeli E, Karnak D, Beder S, Kayacan O, Tutkak H (2008) Diagnostic accuracy of cytokine levels (TNF-alpha, IL-2 and IFN-gamma) in bronchoalveolar lavage fluid of smear-negative pulmonary tuberculosis patients. Respiration 75:73–78
Drobitch RK, Tomilo M, Svensson CK (1992) Immunomodulation and drug acetylation: influence of the immunomodulator tilorone on hepatic, renal and blood N-acetyltransferase activity and on hepatic cytosolic acetyl coenzyme A content. Biochem Pharmacol 43:1643–1648
Acknowledgement
This study was supported by Pharmaceutical Sciences Research Center of Tehran University of Medical Sciences and there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khalili, H., Dashti-Khavidaki, S., Amini, M. et al. Is there any difference between acetylator phenotypes in tuberculosis patients and healthy subjects?. Eur J Clin Pharmacol 66, 261–267 (2010). https://doi.org/10.1007/s00228-009-0745-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-009-0745-1