Abstract
Objectives
To determine how many online clinical trial registers include paediatric trial data, how much information is provided, ease of searching for paediatric trials and the accessibility of paediatric trial data in general.
Methods
Medline and Google were searched for mention of clinical trial registers in July 2008. All registers considered to be eligible were evaluated for trial information provided, search options available, and number of trials, both total and paediatric. A meta-analytic weighted average of the presence of paediatric trials was calculated and compared to the percentage of published paediatric trial articles in Medline. The paediatric trials in the registers were searched for in the World Health Organization's International Clinical Trials Registry Platform (ICTRP). All online, freely accessible registers including ongoing trials on different drugs or therapeutic areas, were eligible for review.
Results
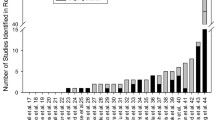
Twelve registers were included in the review. All except one provided detailed trial data, while search options varied between registers: seven provided free-text searching, two listed their trials by condition, two provided elaborate search options (age group, condition, study purpose, etc) and one simply listed its trials. Nine of the 12 registers’ search facilities made it possible to search for paediatric trials, and these were analysed further: the percentage of paediatric trials in the single registers ranged from 4.8 to 33.3%, and the weighted average was 15% (95% confidence interval 8.2–21.8). The percentage of published, paediatric trial articles was 25%. Of the paediatric trials also searched for in the ICTRP, 66% were found.
Conclusions
Great difficulty was found in retrieving paediatric trials due to the limited and inadequate search functions of the registers reviewed but, in general, the registers seem to represent fewer paediatric trials than those reported in the literature. The ICTRP portal is important for trial accessibility, but it is still in an initial phase and far from representative of the global research situation, especially in the field of paediatrics.
Similar content being viewed by others
Abbreviations
- EMEA:
-
European Medicines Agency
- ICMJE:
-
International Committee of Medical Journal Editors
- ICTRP:
-
International Clinical Trials Registry Platform
- MeSH:
-
Medical Subject Headings
References
Bonati M, Pandolfini C, Clavenna A (2004) Disclosure of clinical trials in children. Science 305:1401
Clavenna A, Pandolfini C, Bonati M (2002) Public disclosure of clinical trials in children. Curr Ther Res Clin Exp 63:707–716
Dickersin K, Rennie D (2003) Registering clinical trials. JAMA 290:516–523
Conen D, Torres J, Ridker PM (2008) Differential citation rates of major cardiovascular clinical trials according to source of funding: a survey from 2000 to 2005. Circulation 118:1321–1327
Dowd MD (2004) Breaching the contract: the ethics of nonpublication of research studies. Arch Pediatr Adolesc Med 158:1014–1015
Hartling L, Craig WR, Russell K, Stevens K, Klassen TP (2004) Factors influencing the publication of randomized controlled trials in child health research. Arch Pediatr Adolesc Med 158:983–987
Dwan K, Altman DG, Arnaiz JA, Bloom J, Chan AW, Cronin E et al (2008) Systematic review of the empirical evidence of study publication bias and outcome reporting bias. PLoS ONE 3:e3081
Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R (2008) Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med 358:252–260
Hopewell S, McDonald S, Clarke M, Egger M (2007) Grey literature in meta-analyses of randomized trials of health care interventions. Cochrane Database Syst Rev MR000010
Klassen TP, Wiebe N, Russell K, Stevens K, Hartling L, Craig WR et al (2002) Abstracts of randomized controlled trials presented at the society for pediatric research meeting: an example of publication bias. Arch Pediatr Adolesc Med 156:474–479
Rennie D (2004) Trial registration: a great idea switches from ignored to irresistible. JAMA 292:1359–1362
Dyer O (2004) GlaxoSmithKline faces US lawsuit over concealment of trial results. Br Med J 328:1395
De Angelis C, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R et al (2004) Clinical trial registration: a statement from the International Committee of Medical Journal Editors. N Engl J Med 351:1250–1251
De Angelis C, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R et al (2005) Is this clinical trial fully registered? A statement from the International Committee of Medical Journal Editors. JAMA 293:2927–2929
Reveiz L, Krleza-Jeric K, Chan AW, De AS (2007) Do trialists endorse clinical trial registration? Survey of a Pubmed sample. Trials 8:30
Smyth RL (2001) Research with children. Paediatric practice needs better evidence--gained in collaboration with parents and children. Br Med J 322:1377–1378
Saint Raymond A, Brasseur D (2005) Development of medicines for children in Europe: ethical implications. Paediatr Respir Rev 6:45–51
Caldwell PH, Murphy SB, Butow PN, Craig JC (2004) Clinical trials in children. Lancet 364:803–811
European Parliament and the Council of the European Union (2006) Regulation (EC) No 1902/2006 of the European Parliament and the Council on medicinal products for paediatric use. Publication date 27/12/2006 (L 378/20). Official Journal of the European Union, Brussels
Klassen TP, Hartling L, Craig JC, Offringa M (2008) Children are not just small adults: the urgent need for high-quality trial evidence in children. PLoS Med 5:e172
Pandolfini C, Bonati M (2008) European paediatric research and children's therapeutic needs. A trial review. Acta Paediatr 97:1232–1237
Cohen E, Uleryk E, Jasuja M, Parkin PC (2007) An absence of pediatric randomized controlled trials in general medical journals, 1985–2004. J Clin Epidemiol 60:118–123
(2004) The time is coming [Editorial]. Nat Rev Drug Discov 3:897–898
Zarin DA, Tse T, Ide NC (2005) Trial registration at ClinicalTrials.gov between May and October 2005. N Engl J Med 353:2779–2787
Grobler L, Siegfried N, Askie L, Hooft L, Tharyan P, Antes G (2008) National and multinational prospective trial registers. Lancet 372:1201–1202
Gulmezoglu AM, Pang T, Horton R, Dickersin K (2005) WHO facilitates international collaboration in setting standards for clinical trial registration. Lancet 365:1829–1831
Bonati M, Pandolfini C, Rossi V, Santoro E, de Bolos JM Arnau, Danés Carreras I et al (2004) Launch of a European paediatric clinical trials register. Paediatr Perinat Drug Ther 6:38–39
Bonati M, Pandolfini C (2005) More on compulsory registration of clinical trials: complete clinical trial register is already reality for paediatrics. Br Med J 330:480
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pandolfini, C., Bonati, M. Children’s presence in research. A review of online registers. Eur J Clin Pharmacol 65, 873–880 (2009). https://doi.org/10.1007/s00228-009-0687-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-009-0687-7