Abstract
Purpose
The muscarine receptor antagonist propiverine in immediate release tablet form (IR) undergoes presystemic elimination mediated by CYP450 enzymes and intestinal efflux transporters. The aim of our study with propiverine IR and extended release (ER) was to determine whether propiverine disposition is dose linear, to compare the pharmacokinetics of propiverine in oral solution with IR and ER and to show how absorption rate is associated with bioavailability.
Methods
The pharmacokinetics of propiverine administered as intravenous propiverine (15 mg), 10, 15, and 30 mg propiverine IR, an oral propiverine solution (15 mg) and 10, 15, 30, and 45 mg propiverine ER were measured in two randomized, controlled, single-dose, five-period, cross-over studies, with each case involving a study cohort of ten healthy Caucasian subjects.
Results
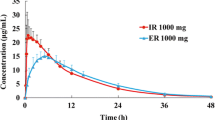
Disposition of propiverine IR and ER was not dose-related. The bioavailability of ER was 64.5 ± 16.1% compared to 50.3 ± 13.4% (non-significant) after administration of the IR and propiverine solution (42.6 ± 14.8%, p < 0.05). The mean absorption time (MAT) of ER (14.2 ± 4.79 h) was significantly longer than that of the solution and IR (3.94 ± 4.14 and 0.38 ± 3.79 h, respectively; both p < 0.05). The bioavailability of propiverine was significantly correlated to the MAT (r = 0.521, p < 0.001). Renal excretion of the metabolite M-23 after propiverine ER administration (6.7 ± 2.7%) was significantly lower than that after administration of the oral solution (10 ± 2.2%) and of IR (9.8 ± 2.7%; both p < 0.05).
Conclusions
The bioavailability of propiverine appears to be dependent on the intestinal site of dissolution and, consequently, on the extent of presystemic intestinal elimination.




Similar content being viewed by others
Abbreviations
- ABCB1:
-
P-glycoprotein
- ABCC2:
-
Multidrug resistance-associated protein 2 (MRP2)
- Propiverine IR:
-
propiverine in immediate release dosage form
- Propiverine ER:
-
propiverine in extended release dosage form
References
Madersbacher H, Murtz G (2001) Efficacy, tolerability and safety profile of propiverine in the treatment of the overactive bladder (non-neurogenic and neurogenic). World J Urol 19:324–335
Michel MC, Hegde SS (2006) Treatment of the overactive bladder syndrome with muscarinic receptor antagonists: a matter of metabolites? Naunyn Schmiedebergs Arch Pharmacol 374:79–85
Wuest M, Hecht J, Christ T et al (2005) Pharmacodynamics of propiverine and three of its main metabolites on detrusor contraction. Br J Pharmacol 145:608–619
Wuest M, Weiss A, Waelbroeck M et al (2006) Propiverine and metabolites: differences in binding to muscarinic receptors and in functional models of detrusor contraction. Naunyn Schmiedebergs Arch Pharmacol 374:87–97
Madersbacher H, Halaska M, Voigt R et al (1999) A placebo-controlled, multicentre study comparing the tolerability and efficacy of propiverine and oxybutynin in patients with urgency and urge incontinence. BJU Int 84:646–651
Stohrer M, Madersbacher H, Richter R et al (1999) Efficacy and safety of propiverine in SCI-patients suffering from detrusor hyperreflexia—a double-blind, placebo-controlled clinical trial. Spinal Cord 37:196–200
Abrams P, Cardozo L, Chapple C et al (2006) Comparison of the efficacy, safety, and tolerability of propiverine and oxybutynin for the treatment of overactive bladder syndrome. Int J Urol 13:692–698
Stohrer M, Murtz G, Kramer G et al (2007) Propiverine compared to oxybutynin in neurogenic detrusor overactivity-results of a randomized, double-blind, multicenter clinical study. Eur Urol 51:235–242
Haustein KO, Huller G (1988) On the pharmacokinetics and metabolism of propiverine in man. Eur J Drug Metab Pharmacokinet 13:81–90
Huller G, Haustein KO, Scheithauer S (1988) Studies on the metabolic pattern of propiverine in urine after single administration. Pharmazie 43:91–95
Wengler A, Schneider T, Zschiesche M et al (1988) Studies on the metabolism of the bladder spasmolytic, propiverin (mictonorm) in humans. Pharmazie 43:652–653
Siegmund W, Nigussie M, Tilahun K et al (1990) Anticholinergic properties of propiverine and its metabolites. Pharmazie 45:67–68
Giessmann T, May K, Moritz KU et al (2004) Disposition of propiverine and its metabolites is influenced by MRP2. Naunyn-Schmiedebergs Arch Pharmacol 369:R156
Kugimiya Y, Hanoaka K, Inada Y (1990) Phase I study of propiverine-hydrochloride. The first report. Single oral dosing study. Jpn J Clin Pharmacol Ther 21:555–565
Siddiqui MA, Perry CM, Scott LJ (2004) Oxybutynin extended-release: a review of its use in the management of overactive bladder. Drugs 64:885–912
van Kerrebroeck P, Kreder K, Jonas U et al (2001) Tolterodine once-daily: superior efficacy and tolerability in the treatment of the overactive bladder. Urology 57:414–421
Mouly S, Paine MF (2003) P-glycoprotein increases from proximal to distal regions of human small intestine. Pharm Res 20:1595–1599
Zimmermann C, Gutmann H, Hruz P et al (2005) Mapping of multidrug resistance gene 1 and multidrug resistance-associated protein isoform 1 to 5 MRNA expression along the human intestinal tract. Drug Metab Dispos 33:219–224
Englund G, Rorsman F, Ronnblom A et al (2006) Regional levels of drug transporters along the human intestinal tract: co-expression of ABC and SLC transporters and comparison with caco-2 cells. Eur J Pharm Sci 29:269–277
Nakamura T, Sakaeda T, Ohmoto N et al (2002) Real-time quantitative polymerase chain reaction for MDR1, MRP1, MRP2, and CYP3A-MRNA levels in caco-2 cell lines, human duodenal enterocytes, normal colorectal tissues, and colorectal adenocarcinomas. Drug Metab Dispos 30:4–6
Zhang QY, Dunbar D, Ostrowska A et al (1999) Characterization of human small intestinal cytochromes P-450. Drug Metab Dispos 27:804–809
Paine MF, Khalighi M, Fisher JM et al (1997) Characterization of interintestinal and intraintestinal variations in human CYP3A-dependent metabolism. J Pharmacol Exp Ther 283:1552–1562
Oertel R, Richter K, Fauler J et al (2002) Increasing sample throughput in pharmacological studies by using dual-column liquid chromatography with tandem mass spectrometry. J Chromatogr A 948:187–192
Oertel R, Kilian B, Siegmund W et al (2006) Determination of propiverine and its metabolites in rat samples by liquid chromatography-tandem mass spectrometry. J Chromatogr A
Richter K, Scheithauer S, Thummler D (1998) High-performance liquid chromatographic determination of propiverine and its N-oxide in human serum. J Chromatogr B Biomed Sci Appl 708:325–329
Aronson JK, Dengler HJ, Dettli L et al (1988) Standardization of symbols in clinical pharmacology. Eur J Clin Pharmacol 35:1–7
Rovner ES, Wein AJ (2002) Once-daily, extended-release formulations of antimuscarinic agents in the treatment of overactive bladder: a review. Eur Urol 41:6–14
Gupta SK, Sathyan G (1999) Pharmacokinetics of an oral once-a-day controlled-release oxybutynin formulation compared with immediate-release oxybutynin. J Clin Pharmacol 39:289–296
Olsson B, Szamosi J (2001) Multiple dose pharmacokinetics of a new once daily extended release tolterodine formulation versus immediate release tolterodine. Clin Pharmacokinet 40:227–235
Massad CA, Kogan BA, Trigo-Rocha FE (1992) The pharmacokinetics of intravesical and oral oxybutynin chloride. J Urol 148:595–597
Chan LM, Lowes S, Hirst BH (2004) The ABCs of drug transport in intestine and liver: efflux proteins limiting drug absorption and bioavailability. Eur J Pharm Sci 21:25–51
Dietrich CG, Geier A, Elferink RPJO (2003) ABC of oral bioavailability: transporters as gatekeepers in the gut. Gut 52:1788–1795
Takano M, Yumoto R, Murakami T (2006) Expression and function of efflux drug transporters in the intestine. Pharmacol Ther 109:137–161
Junemann KP, Hessdorfer E, Unamba-Oparah I et al (2006) Propiverine hydrochloride immediate and extended release: comparison of efficacy and tolerability in patients with overactive bladder. Urol Int 77:334–339
Acknowledgements
The authors are indebted to Hannelore Kreher and Renate Peters for their excellent technical assistance. This work was supported by an institutional grant of APOGEPHA Arzneimittel GmbH Dresden and the German Federal Ministry for Education and Research (grant 01 GG 9845/5, 01 ZZ 0103). Manfred Braeter is an employee of APOGEPHA. All other authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
May, K., Giessmann, T., Wegner, D. et al. Oral absorption of propiverine solution and of the immediate and extended release dosage forms: influence of regioselective intestinal elimination. Eur J Clin Pharmacol 64, 1085–1092 (2008). https://doi.org/10.1007/s00228-008-0528-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-008-0528-0