Abstract
Background
Midazolam (MDZ) is used as an assessment of human cytochrome P450 3A (CYP3A) activity. A single blood measurement is used as a marker of its activity based on an observed correlation between MDZ clearance and the 1′-hydroxymidazolam (1′-OH-MDZ): MDZ plasma ratio is assessed at 0.5 h followig the intake of a single 7.5 mg oral dose of MDZ in healthy young volunteers. In addition, a 4-h plasma MDZ measurement has been found to be an excellent predictor of AUC and CYP3A activity.
Objectives
The main aim of this study was to define a single-point blood sampling in healthy elderly volunteers. The secondary objective was to investigate the pharmacological effects of a low oral dose of MDZ (5 mg) and its potential psychometric changes.
Methods
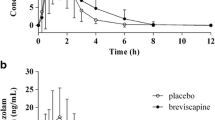
Eight healthy elderly Caucasian volunteers participated in a single-dose, open-label, non-comparative study. Each subject received a single 5 mg oral dose of MDZ. Plasma concentrations of MDZ and its major metabolite, 1′-OH-MDZ, were assayed over 12 h. Secondary assessments of critical flicker fusion (CFF), body sway and mini-mental state examination were also carried out during the 12-h post-administration period.
Results
A moderate correlation was observed between MDZ clearance and the 1′-OH-MDZ: MDZ plasma concentration ratio at 9 h post-dosing (Rho=0.81; p=0.04), but an even better correlation (Rho=0.99; p<0.009) was found between MDZ AUC and MDZ plasma concentration at 6 h post-dosing, with the latter value corresponding approximately to the average mean residence time (MRT) determined in our trial. This study was well-tolerated despite a significant transitory decrease (relative to baseline) in cortical arousal at 1 h post-dosing, as assessed by CFF, and a non-significant decrease (relative to baseline) in balance and vigilance also measured at 1 h and assessed on body sway, compared to baseline values.
Conclusion
Despite the small sample size, based on the results of healthy, elderly volunteers, a single MDZ plasma measurement taken at 6 h post-oral administration may represent an accurate marker of CYP3A phenotype. This single-time-point method could be used safely for predicting drug-drug or diet interactions and identifying individuals with genetic polymorphism that affect CYP3A activity.





Similar content being viewed by others
References
Gorski JC, Jones DR, Haehner-Daniels BD et al (1998) The contribution of intestinal and hepatic CYP3A to the interaction between midazolam and clarithromycin. Clin Pharmacol Ther 64:133–143
Thummel KE, O’Shea D, Paine MF et al (1996) Oral first-pass elimination of midazolam involves both gastrointestinal and hepatic CYP3A-mediated metabolism. Clin Pharmacol Ther 59:491–502
Thummel KE, Shen DD, Podoll TD et al (1994) Use of midazolam as a human cytochrome P450 3A probe: I. In vitro-in vivo correlations in liver transplant patients. J Pharmacol Exp Ther 271:549–556
Carrillo JA, Ramos SI, Agundez JA et al (1998) Analysis of midazolam and metabolites in plasma by high-performance liquid chromatography: probe of CYP3A. Ther Drug Monit 20:319–324
Watkins PB (1994) Noninvasive tests of CYP3A enzymes. Pharmacogenetics 4:171–184
Thummel KE, Shen DD, Podoll TD et al (1994) Use of midazolam as a human cytochrome P450 3A probe: II. Characterization of inter- and intraindividual hepatic CYP3A variability after liver transplantation. J Pharmacol Exp Ther 271:557–566
Thummel KE, Wilkinson GR (1998) In vitro and in vivo drug interactions involving human CYP3A. Annu Rev Pharmacol Toxicol 38:389–430
Lin YS, Lockwood GF, Graham MA et al (2001) In-vivo phenotyping for CYP3A by a single-point determination of midazolam plasma concentration. Pharmacogenetics 11:781–791
Hindmarch I (1982) Critical flicker fusion frequency (CFF): the effects of psychotropic compounds. Pharmacopsychiatry 15:44–48
Patat A, Perault MC, Vandel B et al (1995a) Lack of interaction between a new antihistamine, mizolastine, and lorazepam on psychomotor performance and memory in healthy volunteers. Br J Clin Pharmacol 39:31–38
Patat A, Perault MC, Vandel B et al (1995b) Assessment of the interaction between a partial agonist and a full agonist of benzodiazepine receptors, based on psychomotor performance and memory, in healthy volunteers. J Psychopharmacol 9:91–101
Patat A, Foulhoux P (1985) Effects on postural sway of various benzodiazepine tranquillizers. Br J Clin Pharmacol 2:9–16
McClelland GR (1989) Body sway and the effects of psychoactive drugs-a review. Hum Psychopharmacol 4:3–14
Kapteyn TS, Bles W, Njokiktjien CJ et al (1983) Standardization in platform stabilometry being a part of posturography. Agressologie 24:321–326
Doraiswamy PM, Bieber F, Kaiser L et al (1997) The Alzheimer’s Disease Assessment Scale: patterns and predictors of baseline cognitive performance in multicenter Alzheimer’s disease trials. Neurology 48:1511–1517
Folstein MF, Folstein SE, McHugh PR et al (1975) “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Gagnon M, Letenneur L, Dartigues JF et al (1990) Validity of the Mini-Mental State examination as a screening instrument for cognitive impairment and dementia in French elderly community residents. Neuroepidemiology 9:143–150
Smith MT, Heazlewood V, Eadie MJ et al (1984) Pharmacokinetics of Midazolam in the Aged. Eur J Clin Pharmacol 26:381–388
Mandema JW, Tuk B, van Steveninck AL et al (1992) Pharmacokinetic-pharmacodynamic modeling of the central nervous system effects of midazolam and its main metabolite alpha-hydroxymidazolam in healthy volunteers. Clin Pharmacol Ther 51:715–728
Villeneuve JP, L’Ecuyer L, De Maeght S et al (2000) Prediction of cyclosporine clearance in liver transplant recipients by the use of midazolam as a cytochrome P450 3A probe. Clin Pharmacol Ther 67:242–248
Paine MF, Shen DD, Kunze KL et al (1996) First-pass metabolism of midazolam by the human intestine. Clin Pharmacol Ther 60:14–24
Dundee JW, Halliday NJ, Harper KW et al (1984) Midazolam: a review of its pharmacological properties and therapeutic use. Drugs 28:519–543
Greenblatt DJ, Abernethy DR, Locniskar A et al (1984) Effect of age, gender and obesity on midazolam kinetics. Anesthesiology 61:27–35
Acknowledgements
This study was supported by the Délégation Régionale de la Recherche Clinique de la Région Poitou-Charentes, and conducted at the Clinical Research Center of Poitiers’ University Hospital, France. We thank Ms. Stephanie Ragot for her help in performing statistical analysis of demographic and pharmacodynamic data.
Author information
Authors and Affiliations
Corresponding author
Additional information
In memoriam: S. Bouquet
Rights and permissions
About this article
Cite this article
Krupka, E., Venisse, N., Lafay, C. et al. Probe of CYP3A by a single-point blood measurement after oral administration of midazolam in healthy elderly volunteers. Eur J Clin Pharmacol 62, 653–659 (2006). https://doi.org/10.1007/s00228-006-0159-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-006-0159-2