Abstract
Objective
The objective of the present investigation was to develop a population pharmacodynamic model for midazolam- and lorazepam-induced sedation upon long-term continuous infusion in critically ill patients.
Methods
The study was conducted in 59 patients receiving lorazepam and 54 patients receiving midazolam by continuous infusion for at least 24 h. Repeated blood samples were obtained for determination of the concentrations of lorazepam and midazolam. The level of sedation was assessed using a 5-point sedation scale.
Results
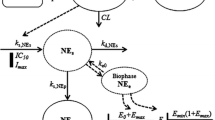
The pharmacokinetics of lorazepam and midazolam was described with previously proposed pharmacokinetic models. For the pharmacodynamics, the probability that the sedation was equal to or more than a specific score was described using a sigmoid Emax model. The EC50 values of lorazepam for the sedation scores equal or larger than 2–5 were 6.1, 57, 184 and 529 ng/ml, respectively. The corresponding values for midazolam were 216, 483, 1100 and 2200 ng/ml. Inter-individual variability in the EC50 values was relatively high with a CV of 68% for lorazepam and 86% for midazolam (p=0.035). No covariates explaining part of the observed inter-individual variability were identified.
Conclusion
The population pharmacodynamic model shows a similarly wide intra- and inter-individual variability in the pharmacodynamics of both lorazepam and midazolam. Thus, the previously observed differences in “ease of titration” between lorazepam and midazolam are unrelated to pharmacodynamic factors.



Similar content being viewed by others
References
Swart EL, Strack van Schijndel RJM, van Loenen AC, Thijs LG (1999) Continuous infusion of lorazepam versus midazolam in patients in the intensive care unit: sedation with lorazepam is easier to manage and is more cost-effective. Crit Care Med 27:1461–1465
Swart EL, Zuideveld KP, de Jongh J, Danhof M, Thijs LG, Strack van Schijndel RJM (2004) Comparative population pharmacokinetics of lorazepam and midazolam upon long-term continuous infusion in critically ill patients. Br J Clin Pharmacol 57:135–145
Mandema JW, Tuk B, van Steveninck AL, Breimer DD, Cohen AF, Danhof M (1992) Pharmacokinetic-pharmacodynamic modeling of the central nervous system effects of midazolam and its main metabolite α-hydroxymidazolam in healthy volunteers. Clin Pharmacol Ther 51:715–728
Barr J, Zomorodi K, Bertaccini EJ, Shafer SL, Geller E (2001) A double-blind, randomized comparison of IV lorazepam versus midazolam for sedation of ICU patients via a pharmacologic model. Anesthesiology 95:286–298
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
O’Sullivan G, Park GR (1990) The assessment of sedation. J Clin Intensive Care 1:116–121
Hansen-Flaschen J, Cowen J, Polomano R (1994) Beyond the Ramsay scale: need for a validated measure of sedation drug efficacy in the intensive care unit. Crit Care Med 22:732–733
Haberthür C, Lehmann F, Ritz R (1996) Assessing of depth of midazolam sedation using objective parameters. Intensive Care Med 2:1385–1390
Schulte-Tamburen AM, Scheier J, Briegel J, Schwender D, Peter K (1999) Comparison of five sedation scoring systems by means of auditory evoked potentials. Intensive Care Med 25:377–382
De Jonghe B, Cook D, Appere-De Vecchi C, Guyatt G, Meade M, Outin H (2000) Using and understanding sedation scoring systems: a systematic review. Intensive Care Med 26:275–285
Vletter AA, Burm AGL, Breimer LTM, Spierdijk J (1990) High performance liquid chromatographic assay of midazolam and flumazenil simultaneously or individually in human plasma. J Chromatogr Biomed Appl 530:177–185
Shafer SL, Varvel JR, Aziz N, Scott JC (1990) Pharmacokinetics of fentanyl administered by computer-controlled infusion pump. Anesthesiology 73:1091–1102
Somma J, Donner A, Zomorodi K, Sladen R, Ramsay J, Geller E, Shafer SL (1998) Population pharmacodynamics of midazolam administered by target controlled infusion in SICU patients after CABG surgery. Anesthesiology 89:1430–1443
Shelly MP, Sultan MA, Bodenham A, Park GR (1991) Midazolam infusions in critically ill patients. Eur J Anaesthesiol 8:21–27
Bodenham A, Shelly MP, Park GR (1988) The altered pharmacokinetics and pharmacodynamics of drugs commonly used in critically ill patients. Clin Pharmacokinet 14:347–373
Schoch P, Moreau JL, Martin JR, Haefely WE (1993) Aspects of benzodiazepine receptor structure and function with relevance to drug tolerance and dependence. Biochem Soc Symp 59:121–134
Miller LG, Greenblatt DJ, Barnhill JG, Shader RI (1998) Chronic benzodiazepine administration I. Tolerance is associated with benzodiazepine receptor downregulation and decreased γ–aminobutyric acid receptor function. J Pharmacol Exp Ther 246:170-176
Vinik HR, Bradley EL, Kissin I (1989) Midazolam-alfentanil synergism for anesthetic induction in patients. Anesth Analg 69:213–217
Kissin I, Vinik HR, Castillo R, Bradleu EL (1990) Alfentanil potentiates midazolam-induced unconsciousness in subanalgesic doses. Anesth Analg 71:65–69
Vinik HR, Bradley EL, Kissin I (1994) Triple anesthetic combination: propofol-midazolam-alfentanil. Anesth Analg 78:354–358
Boulieu R, Lehmann B, Salord F, Fisher C, Morlet D (1998) Pharmacokinetics of midazolam and its main metabolite 1-hydroxymidazolam in intensive care patients. Eur J Drug Metab Pharmacokinet 23:255–258
Bauer TM, Ritz R, Haberthür C, Ha HR, Hunkeler W, Sleight AJ, Scollo-Lavizzari G, Haefeli WE (1995) Prolonged sedation due to accumulation of conjugated metabolites of midazolam. Lancet 346:145–147
Driessen JJ, Vree TB, Guelen PJM (1991) The effects of acute changes in renal function on the pharmacokinetics of midazolam during long-term infusion in ICU patients. Acta Anaesthesiol Belg 42:49–55
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Swart, E.L., Zuideveld, K.P., de Jongh, J. et al. Population pharmacodynamic modelling of lorazepam- and midazolam-induced sedation upon long-term continuous infusion in critically ill patients. Eur J Clin Pharmacol 62, 185–194 (2006). https://doi.org/10.1007/s00228-005-0085-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-005-0085-8