Abstract
Objective
We sought to quantify the influence of the CYP3A inhibitor erythromycin on the pharmacokinetics of everolimus, a CYP3A substrate.
Methods
This was a two-period, single-sequence, crossover study in 16 healthy subjects. In period 1, subjects received the reference treatment of a single 2-mg dose of everolimus. In period 2, they received the test treatment of erythromycin 500 mg three times daily for a total of 9 days and a single 2-mg dose of everolimus coadministered on the fifth day of erythromycin therapy. The test/reference ratio and 90% confidence interval (CI) were derived for everolimus C max and AUC.
Results
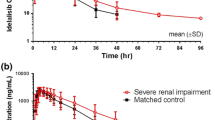
During erythromycin coadministration, everolimus C max increased 2.0-fold (90% CI, 1.8–2.3) from 20±5 ng/ml to 40±10 ng/ml. Everolimus AUC increased 4.4-fold (90% CI, 3.5–5.4) from 116±37 ng h/ml to 524±225 ng h/ml. Everolimus half-life was prolonged by 39% from 32±6 h to 44±6 h. Erythromycin predose concentrations were not changed after single-dose administration of everolimus.
Conclusion
Multiple-dose erythromycin increased single-dose everolimus blood levels by an average 4.4-fold (range, 2.0–12.6). During erythromycin treatment, a compensatory everolimus dose reduction should be made guided by everolimus therapeutic drug monitoring.



Similar content being viewed by others
References
Kovarik JM (2004) Everolimus: a proliferation signal inhibitor targeting primary causes of allograft dysfunction. Drugs Today 40:101–109
Kovarik JM, Tedesco H, Pascual J (2004) Everolimus therapeutic concentration range defined from a prospective trial with reduced-exposure cyclosporine in de novo kidney transplantation Ther Drug Monit 26(5):499–505
Kovarik JM, Hsu CH, McMahon L (2001) Population pharmacokinetics of everolimus in de novo renal transplant patients: impact of ethnicity and comedications. Clin Pharmacol Ther 70:247–254
Brignol N, McMahon L, Luo S (2001) High-throughput semi-automated 96-well liquid/liquid extraction and liquid chromatography/mass spectrometry analysis of everolimus(RAD001) and cyclosporine A(CsA) in whole blood. Rapid Commun Mass Spectrom 15:898–907
Danan G, Deschatoire V, Pessayre D (1981) Self-induction by erythromycin of its own transformation into a metabolite forming an inactive complex with reduced cytochrome P-450. J Pharmacol Exp Ther 218:509–514
Pai MP, Graci DM, Amsden GW (2000) Macrolide drug interactions: an update. Ann Pharmacother 34:495–513
Bjornsson TD, Callaghan JT, Einolf HJ (2003) Conduct of in vitro and in vivo drug-drug interaction studies: a PhRMA perspective. J Clin Pharmacol 43:443–469
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was financed by Novartis Pharmaceuticals.
Rights and permissions
About this article
Cite this article
Kovarik, J., Beyer, D., Bizot, M. et al. Effect of multiple-dose erythromycin on everolimus pharmacokinetics. Eur J Clin Pharmacol 61, 35–38 (2005). https://doi.org/10.1007/s00228-004-0866-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-004-0866-5