Abstract
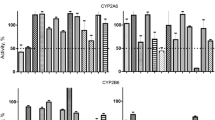
Objective: The aim of the present study was to predict the drug interaction potential of memantine by elucidation of its inhibitory effects on cytochrome P450 enzymes using pooled human liver microsomes (HLM) and recombinant P450s. Methods: The inhibitory potency of memantine on CYP1A2, CYP2A6, CYP2B6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4 activities was examined with specific probe drugs in HLM and recombinant P450s. The in vivo drug interactions of memantine were predicted in vitro using the [I ]/([I ] + KI) values. Results: In HLM, memantine inhibited CYP2B6 and CYP2D6 activities, with KI (IC50) values of 76.7 (279.7) and 94.9 (368.7) μM, respectively. Both inhibitions were competitive. In addition, cDNA-expressed P450s were used to confirm these results. Memantine strongly inhibited recombinant CYP2B6 activity with IC50 (KI) value of 1.12 (0.51) μM and activity of recombinant CYP2D6 with IC50 (KI) value of 242.4 (84.4) μM. With concentrations up to 1,000 μM, memantine showed no appreciable effect on CYP1A2, CYP2E1, CYP2C9, or CYP3A4 activities and a slight decrease of CYP2A6 and CYP2C19 activities. Based on [I ]/([I ] + KI) values calculated using peak total plasma concentration (or enzyme-available concentration in the liver) of memantine and the KI obtained in HLM, 1.3 (13.5), and 1.0% (11.2%), inhibition of the clearance of CYP2B6 and CYP2D6 substrates could be expected, respectively. Nevertheless, when considering KI values obtained from cDNA-expressed CYP2B6, as generally recommended, even 66.2% (95.9%) decrease in metabolism of coadministered CYP2B6 substrates could be anticipated. Conclusion: Memantine exerts selective inhibition of CYP2B6 activity at clinically relevant concentrations, suggesting the potential for clinically significant drug interactions. Inhibition of other CYPs during memantine therapy is unlikely. Moreover, memantine represents a new, potent, selective inhibitor of recombinant CYP2B6, which may prove useful for screening purposes during early phases of in vitro drug metabolism studies with new chemical entities.


Similar content being viewed by others
References
Le DA, Lipton SA (2001) Potential and current use of N-methyl-D-aspartate (NMDA) receptor antagonists in diseases of aging. Drugs Aging 18:717–724
Doraiswamy PM (2002) Non-cholinergic strategies for treating and preventing Alzheimer’s disease. CNS Drugs 16:811–824
Jarvis B, Figgitt D (2003) Memantine. Drugs Aging 20:465–476
Collins ED, Ward AS, McDowell DM, Foltin RW, Fischman MW (1998) The effects of memantine on the subjective, reinforcing and cardiovascular effects of cocaine in humans. Behav Pharmacol 9:587–598
Wesemann W, Sontag KH, Maj J (1983) Pharmacodynamics and pharmacokinetics of memantine. Arzneimittelforschung 33:1122–1134
Freudenthaler S, Meineke I, Schreeb KH, Boakye E, Gundert-Remy U, Gleiter CH (1998) Influence of urine pH and urinary flow on the renal excretion of memantine. Br J Clin Pharmacol 46:541–546
Axura (2002) European public assessment report (EPAR). The European Agency for the Evaluation of Medicinal Products, London. (http://www.emea.eu.int/)
Kornhuber J, Weller M, Schoppmeyer K, Riederer P (1994) Amantadine and memantine are NMDA receptor antagonists with neuroprotective properties. J Neural Transm Suppl 43:91–104
Weaver RJ (2001) Assessment of drug–drug interactions: concepts and approaches. Xenobiotica 31:499–538
Lupp A, Kerst S, Karge E, Quack G, Klinger W (1998) Investigation on possible antioxidative properties of the NMDA-receptor antagonists ketamine, memantine, and amantadine in comparison to nicanartine in vitro. Exp Toxicol Pathol 50:501–506
Lupp A, Kerst S, Karge E (2003) Evaluation of possible pro- or antioxidative properties and of the interaction capacity with the microsomal cytochrome P450 system of different NMDA-receptor ligands and of taurine in vitro. Exp Toxicol Pathol 54:441–448
Belanger PM, Grech-Belanger O, Labadie R (1979) Depression of hepatic microsomal enzyme systems by amantadine hydrochloride in the rat. J Pharmacol Exp Ther 211:4854–4890
Stiborova M, Borek-Dohalska L, Hodek P, Mraz J, Frei E (2002) New selective inhibitors of cytochromes P450 2B and their application to antimutagenesis of tamoxifen. Arch Biochem Biophys 403:41–49
Burke MD, Prough RA, Mayer RT (1977) Characteristics of a microsomal cytochrome P-448-mediated reaction. Ethoxyresorufin O-de-ethylation. Drug Metab Dispos 5:1–8
Aitio A (1978) A simple and sensitive assay of 7-ethoxycoumarin deethylation. Anal Biochem 85:488–491
Gervot L, Rochat B, Gautier JC, Bohnenstengel F, Kroemer H, de Berardinis V, Martin H, Beaune P, de Waziers I (1999) Human CYP2B6: expression, inducibility and catalytic activities. Pharmacogenetics 9:295–306
Knodell RG, Hall SD, Wilkinson GR, Guengerich FP (1987) Hepatic metabolism of tolbutamide: characterization of the form of cytochrome P-450 involved in methyl hydroxylation and relationship to in vivo disposition. J Pharmacol Exp Ther 241:1112–1119
Lam YW, Rodriguez SY (1993) High-performance liquid chromatography determination of dextromethorphan and dextrorphan for oxidation phenotyping by fluorescence and ultraviolet detection. Ther Drug Monit 15:300–304
Wrighton SA, Stevens JC, Becker GW, VandenBranden M (1993) Isolation and characterization of human liver cytochrome P450 2C19: correlation between 2C19 and S-mephenytoin 4′-hydroxylation. Arch Biochem Biophys 306:240–245
Lucas D, Berthou F, Girre C, Poitrenaud F, Menez JF (1993) High-performance liquid chromatographic determination of chlorzoxazone and 6-hydroxychlorzoxazone in serum: a tool for indirect evaluation of cytochrome P4502E1 activity in humans. J Chromatogr 622:79–86
Guengerich FP, Martin MV, Beaune PH, Kremers P, Wolff T, Waxman DJ (1986) Characterization of rat and human liver microsomal cytochrome P-450 forms involved in nifedipine oxidation, a prototype for genetic polymorphism in oxidative drug metabolism. J Biol Chem 261:5051–5060
Dixon M (1953) The determination of enzyme inhibitor constants. Biochem J 55:170–171
Bourrie M, Meunier V, Berger Y, Fabre G (1996) Cytochrome P450 isoform inhibitors as a tool for the investigation of metabolic reactions catalyzed by human liver microsomes. J Pharmacol Exp Ther 277:321–332
Hickman D, Wang JP, Wang Y, Unadkat JD (1998) Evaluation of the selectivity of in vitro probes and suitability of organic solvents for the measurement of human cytochrome P450 monooxygenase activities. Drug Metab Dispos 26:207–215
Segel IH (1975) Enzyme kinetics: behavior and analysis of rapid equilibrium and steady-state enzyme systems. Wiley, New York
Cornish-Bowden A (1974) A simple graphical method for determining the inhibition constants of mixed, uncompetitive, and non-competitive inhibitors. Biochem J 137:143–144
Cortes A, Cascante M, Cardenas ML, Cornish-Bowden A (2001). Relationships between inhibition constants, inhibitor concentrations for 50% inhibition and types of inhibition: new ways of analysing data. Biochem J 357:263–268
Samnick S, Ametamey S, Leenders KL, Vontobel P, Quack G, Parsons CG, Neu H, Schubiger PA (1998) Electrophysiological study, biodistribution in mice, and preliminary PET evaluation in a rhesus monkey of 1-amino-3-[18F]fluoromethyl-5-methyl-adamantane (18F-MEM): a potential radioligand for mapping the NMDA-receptor complex. Nucl Med Biol 25:323–330
Eagling VA, Tjia JF, Back DJ (1998) Differential selectivity of cytochrome P450 inhibitors against probe substrates in human and rat liver microsomes. Br J Clin Pharmacol 45:107–114
Zhang W, Kilicarslan T, Tyndale RF, Sellers EM (2001) Evaluation of methoxsalen, tranylcypromine, and tryptamine as specific and selective CYP2A6 inhibitors in vitro. Drug Metab Dispos 29:897–902
Hesse LM, von Moltke LL, Shader RI, Greenblatt DJ (2001) Ritonavir, efavirenz, and nelfinavir inhibit CYP2B6 activity in vitro: potential drug interactions with bupropion. Drug Metab Dispos 29:100–102
Rae JM, Soukhova NV, Flockhart DA, Desta Z (2002) Triethylenethiophosphoramide is a specific inhibitor of cytochrome P450 2B6: implications for cyclophosphamide metabolism. Drug Metab Dispos 30:525–530
Fan PW, Gu C, Marsh SA, Stevens JC (2003) Mechanism-based inactivation of cytochrome P450 2B6 by a novel terminal acetylene inhibitor. Drug Metab Dispos 31:28–36
Jushchyshyn MI, Kent UM, Hollenberg PF (2003) The mechanism-based inactivation of human cytochrome P450 2B6 by phencyclidine. Drug Metab Dispos 31:46–52
Mimura M, Baba T, Yamazaki H, Ohmori S, Inui Y, Gonzalez FJ, Guengerich FP, Shimada T (1993) Characterization of cytochrome P-450 2B6 in human liver microsomes. Drug Metab Dispos 21:1048–1056
Code EL, Crespi CL, Penman BW, Gonzalez FJ, Chang TK, Waxman DJ (1997) Human cytochrome P4502B6: interindividual hepatic expression, substrate specificity, and role in procarcinogen activation. Drug Metab Dispos 25:985–993
Cawley GF, Zhang S, Kelley RW, Backes WL (2001) Evidence supporting the interaction of CYP2B4 and CYP1A2 in microsomal preparations. Drug Metab Dispos 29:1529–1534
Ekins S, Wrighton SA (1999) The role of CYP2B6 in human xenobiotic metabolism. Drug Metab Rev 31:719–754
Chang TK, Weber GF, Crespi CL, Waxman DJ (1993) Differential activation of cyclophosphamide and ifosphamide by cytochromes P-450 2B and 3A in human liver microsomes. Cancer Res 53:5629–5637
White IN, De Matteis F, Gibbs AH, Lim CK, Wolf CR, Henderson C, Smith LL (1995) Species differences in the covalent binding of [14C]tamoxifen to liver microsomes and the forms of cytochrome P450 involved. Biochem Pharmacol 49:1035–1042
Heyn H, White RB, Stevens JC (1996) Catalytic role of cytochrome P450 2B6 in the N-demethylation of S-mephenytoin. Drug Metab Dispos 24:948–954
Ono S, Hatanaka T, Miyazawa S, Tsutsui M, Aoyama T, Gonzalez FJ, Satoh T (1996) Human liver microsomal diazepam metabolism using cDNA-expressed cytochrome P450s: role of CYP2B6, 2C19 and the 3A subfamily. Xenobiotica 26:1155–1166
Aoyama T, Yamano S, Guzelian PS, Gelboin HV, Gonzalez FJ (1990) Five of 12 forms of vaccinia virus-expressed human hepatic cytochrome P450 metabolically activate aflatoxin B1. Proc Natl Acad Sci U S A 87:4790–4793
Shou M, Korzekwa KR, Krausz KW, Buters JT, Grogan J, Goldfarb I, Hardwick JP, Gonzalez FJ, Gelboin HV (1996) Specificity of cDNA-expressed human and rodent cytochrome P450s in the oxidative metabolism of the potent carcinogen 7,12-dimethylbenz[a]anthracene. Mol Carcinog 17:241–249
Shibutani S, Ravindernath A, Suzuki N, Terashima I, Sugarman SM, Grollman AP, Pearl ML (2000) Identification of tamoxifen-DNA adducts in the endometrium of women treated with tamoxifen. Carcinogenesis 21:1461–1467
Schmider J, von Moltke LL, Shader RI, Harmatz JS, Greenblatt DJ (1999) Extrapolating in vitro data on drug metabolism to in vivo pharmacokinetics: evaluation of the pharmacokinetic interaction between amitriptyline and fluoxetine. Drug Metab Rev 31:545–560
Acknowledgements
We gratefully acknowledge the skillful technical assistance of Jitka Hajkova. This study was performed within the framework of the COST B15.30 (European Co-operation in the Field of Scientific and Technical Research) project. The present study complies with the current laws of the Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Micuda, S., Mundlova, L., Anzenbacherova, E. et al. Inhibitory effects of memantine on human cytochrome P450 activities: prediction of in vivo drug interactions. Eur J Clin Pharmacol 60, 583–589 (2004). https://doi.org/10.1007/s00228-004-0825-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-004-0825-1