Abstract
Objective
To evaluate the gastrointestinal safety of cyclo-oxygenase-2 inhibitors under their real conditions of use.
Design
Case/non-case study.
Setting
Adverse drug reactions (ADRs) in adults recorded in the French Pharmacovigilance Database between 25 May 2000 and 31 December 2002.
Materials
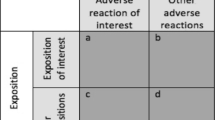
Cases were all reports of “serious” oeso-gastro-duodenal ADRs (oeso-gastro-duodenal ulcers, oesophagitis, gastritis, duodenitis). Five non-cases were randomly selected for one case from all other non oeso-gastro-duodenal reports in the database after matching them for age, gender and period of occurrence.
Analysis
Coxib exposure was compared among cases and non-cases, with adjustment for matching factors: French Regional Pharmacovigilance Centres that collected ADRs, reporter health professional’s characteristics and exposures to non-selective non-steroidal anti-inflammatory, aspirin, anticoagulant, antiplatelet and gastroprotective drugs.
Results
Included in the study were 505 cases and 2,525 non-cases. A positive association was found between occurrence of oeso-gastro-duodenal ADRs and coxib (adjusted odds ratio 14.9 [95% CI 9.3–23.7]), diclofenac (9.2 [3.8–22.2]), ibuprofen (7.3 [3.2–16.6]) or oxicam (25.3 [11.9–53.6]) use.
Conclusion
Despite the compulsory limits of the case/non-case methodology, the present study shows that coxibs did induce “serious” gastrointestinal ADRs in real clinical practice. These results underline the need for pharmacoepidemiological studies under real conditions of use in order to verify (or not) the conclusions of clinical trials.
Similar content being viewed by others
References
Montastruc JL (2003) Pharmacology: selective for whom? Marketing ploy. Prescrire Int 12:119
Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz MB, Hawkey CJ, Hochberg MC, Kvien TK, Schnitzer TJ, VIGOR Study group (2000) Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med 343:1520–1528
Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, Makuch R, Eisen G, Agrawal NM, Stenson WF, Burr AM, Zhao WW, Kent JD, Lefkowith JB, Verburg KM, Geis GS (2000) Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA 284:1247–1255
Layton D, Riley J, Wilton LV, Shakir SA (2003) Safety profile of rofecoxib as used in general practice in England: results of a prescription-event monitoring study. Br J Clin Pharmacol 55:166–174
WHO (2000) Celecoxib: adverse reaction reports. WHO Drug Inf 14:93–94
Egberts AC, Meyboom RH, De Koning FH, Bakker A, Leufkens HG (1997) Non-puerperal lactation associated with antidepressant drug use. Br J Clin Pharmacol 44:277–281
Moore N, Kreft-Jais C, Haramburu F, Noblet C, Andrejak M, Ollagnier M, Begaud B (1998) Reports of hypoglycaemia associated with the use of ACE inhibitors and other drugs: a case/non-case study in the French pharmacovigilance system database. Br J Clin Pharmacol 44:513–518
Egberts AC, Meyboom RH, van Puijenbroek EP (2002) Use of measures of disproportionality in pharmacovigilance: three Dutch examples. Drug Saf 25:453–458
Stricker BH, Tijssen JG (1992) Serum sickness-like reactions to cefaclor. J Clin Epidemiol 45:1177–1184
Moore N, Noblet C, Kreft-Jais C, Lagier G, Ollagnier M, Imbs JL (1995) French pharmacovigilance database system: examples of utilisation. Therapie 50:557–562
WHO (1992) International monitoring of adverse reactions to drugs: adverse reaction terminology. WHO collaborating Centre for International Drug Monitoring, Uppsala
Edwards IR, Aronson JK (2000) Adverse drug reactions: definitions, diagnosis, and management. Lancet 356:1255–1259
Langman MJ, Jensen DM, Watson DJ, Harper SE, Zhao PL, Quan H, Bolognese JA, Simon TJ (1999) Adverse upper gastrointestinal effects of rofecoxib compared with NSAIDs. JAMA 282:1929–1933
Begaud B, Evreux JC, Jouglard J, Lagier G (1985) Imputation of the unexpected or toxic effects of drugs. Actualization of the method used in France. Therapie 40:111–118
MacDonald TM, Morant SV, Goldstein JL, Burke TA, Pettitt D (2003) Channelling bias and the incidence of gastrointestinal haemorrhage in users of meloxicam, coxibs, and older, non-specific non-steroidal anti-inflammatory drugs. Gut 52:1265–1270
Van der Heijden PG, van Puijenbroek EP, van Buuren S, van der Hofstede JW (2002) On the assessment of adverse drug reactions from spontaneous reporting systems: the influence of under-reporting on odds ratios. Stat Med 21:2027–2044
Desboeuf K, Lapeyre-Mestre M, Montastruc JL (1998) Risk of gastrointestinal haemorrhage with calcium antagonists. Br J Clin Pharmacol 46:87–89
Wilson AM, Thabane L, Holbrook A (2004) Application of data mining data in pharmacovigilance. Br J Clin Pharmacol 57:127–134
Henry D, Dobson A, Turner C (1993) Variability in the risk of major gastrointestinal complications from nonaspirin nonsteroidal anti-inflammatory drugs. Gastroenterology 105:1078–1088
Garcia-Rodriguez L, Hernandez-Diaz S (2001) Relative risk of upper gastrointestinal complications among users of acetaminophen and nonsteroidal anti-inflammatory drugs. Epidemiology 12:570–576
Mamdani M, Rochon PA, Juurlink DN, Kopp A, Anderson GM, Naglie G, Austin J, Laupacis A (2002) Observational study of upper gastrointestinal haemorrhage in elderly patients given selective cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs. BMJ 325:624
Laporte JR, Ibanez L, Vidal X, Vendrell L, Leone R (2004) Upper gastrointestinal bleeding associated with the use of NSAIDs: newer versus older agents. Drug Saf 27:411–420
Mamdani M, Juurlink DN, Kopp A, Naglie G, Austin PC, Laupacis A (2004) Gastrointestinal bleeding after the introduction of COX 2 inhibitors: ecological study. BMJ 328:1415–1416
Chan CC, Boyce S, Brideau C, Charleson S, Cromlish W, Ethier D, Evans J, Ford-Hutchinson AW, Forrest MJ, Gauthier JY, Gordon R, Gresser M, Guay J, Kargman S, Kennedy B, Leblanc Y, Leger S, Mancini J, O’ Neill GP, Ouellet M, Patrick D, Percival MD, Perrier H, Prasit P, Rodger I et al (1999) Rofecoxib [Vioxx, MK-0966; 4-(4′-methylsulfonylphenyl)-3-phenyl-2-(5H)-furanone]: a potent and orally active cyclooxygenase-2 inhibitor. Pharmacological and biochemical profiles. J Pharmacol Exp Ther 290:551–560
Laporte JR, Carne X, Vidal X, Moreno V, Juan J (1991) Upper gastrointestinal bleeding in relation to previous use of analgesics and non-steroidal anti-inflammatory drugs. Catalan Countries Study on Upper Gastrointestinal Bleeding. Lancet 337:85–89
Garcia Rodriguez LA, Jick H (1994) Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet 343:769–772
Acknowledgements
We acknowledge the French network of the Pharmacovigilance Centres for giving us their observations and Doctors Pascal Auriche and Carmen Kreft-Jais (AFSSAPS) for helping us to extract data from the French Pharmacovigilance Database. Contributors: S.L., M.L.M., and J.L.M. analysed and wrote up the study. J.L.M. corrected the text and is the guarantor. Funding: Grant by the French Ministry of Health. Competing interests: None.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Lugardon, S., Lapeyre-Mestre, M., Montastruc, J.L. et al. Upper gastrointestinal adverse drug reactions and cyclo-oxygenase-2 inhibitors (celecoxib and rofecoxib): a case/non-case study from the French Pharmacovigilance Database. Eur J Clin Pharmacol 60, 673–677 (2004). https://doi.org/10.1007/s00228-004-0813-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-004-0813-5