Abstract
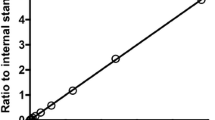
Objective: Propofol (2,6-diisopropylphenol) is widely used for anesthetic induction as well as for chronic sedation in intensive care units. In this study, we investigated the interaction between propofol and premedications, i.e., psychotropic and antianxiety agents (diazepam, midazolam), hypnotics (thiamylal), local anesthetics (lidocaine), depolarizing muscular relaxants (vecuronium), an antihypertensive (clonidine) and an H2-receptor antagonist (cimetidine) using human liver microsomes in vitro. Methods: The interaction effects between propofol and premedications were examined using human liver microsomal preparation in vitro. The concentration of propofol was determined by HPLC with UV detection. Results: The apparent Michaelis–Menten constant (Km) and the maximal velocity of total metabolic formation (Vmax) of propofol in human liver microsomes were 123 μM and 26.1 μmol/min per milligram of mg protein, respectively. Seven premedications (diazepam, midazolam, thiamylal, lidocaine, cimetidine, vecuronium, and clonidine) did not inhibit propofol metabolism in human liver microsomes at concentrations within the therapeutic range. Conclusions: These results showed no interactions between propofol and seven premedication drugs within the therapeutic range of propofol using human liver microsomes in vitro.

Similar content being viewed by others
References
Bryson HM, Fulton BR, Faulds D (1995) Propofol An update of its use in anaesthesia and conscious sedation. Drugs 50:513–559
Baker MT, Chadam MV, Ronnenberg WC Jr (1993) Inhibitory effects of propofol on cytochrome P450 activities in rat hepatic microsomes. Anesth Analg 76:817–821
Chen TL, Ueng TH, Chen SH, Lee PH, Fan SZ, Liu CC (1995) Human cytochrome P450 mono-oxygenase system is suppressed by propofol. Br J Anaesth 74:558–562
Janicki PK, James MF, Erskine WA (1992) Propofol inhibits enzymatic degradation of alfentanil and sufentanil by isolated liver microsomes in vitro. Br J Anaesth 68:311–312
Leung BP, Miller E, Park GR (1997) The effect of propofol on midazolam metabolism in human liver microsome suspension. Anaesthesia 52:945–948
McKillop D, Wild MJ, Butters CJ, Simcock C (1998) Effects of propofol on human hepatic microsomal cytochrome P450 activities. Xenobiotica 28:845–853
Hamaoka N, Oda Y, Hase I, Mizutani K, Nakamoto T, Ishizaki T, Asada A (1999) Propofol decreases the clearance of midazolam by inhibiting CYP3A4: an in vivo and in vitro study. Clin Pharmacol Ther 66:110–107
Mertens MJ, Vuyk J, Olofsen E, Bovill JG, Burm AG (2001) Propofol alters the pharmacokinetics of alfentanil in healthy male volunteers. Anesthesiology 94:949–957
Schulz M, Schmoldt A (2003) Therapeutic and toxic blood concentrations of more than 800 drugs and other xenobiotics. Pharmazie 58:447–474
Rae JM, Soukhova NV, Flockhart DA, Desta Z (2002) Triethylenethiophosphoramide is a specific inhibitor of cytochrome P450 2B6: implications for cyclophosphamide metabolism. Drug Metab Dispos 30:525–530
Oda Y, Hamaoka N, Hiroi T, Imaoka S, Hase I, Tanaka K, Funae Y, Ishizaki T, Asada A (2001) Involvement of human liver cytochrome P4502B6 in the metabolism of propofol. Br J Clin Pharmacol 51:281–285
Markowitz JS, Wells BG, Carson WH (1995) Interactions between antipsychotic and antihypertensive drugs. Ann Pharmacother 29:603–609
Vanden Bossche H, Koymans L, Moereels H (1995) P450 inhibitors of use in medical treatment: focus on mechanisms of action. Pharmacol Ther 67:79–100
Murray M (1997) Drug-mediated inactivation of cytochrome P450. Clin Exp Pharmacol Physiol 24:465–470
Tanaka E (1998) Clinically important pharmacokinetic drug-drug interactions: role of cytochrome P450 enzymes. J Clin Pharm Ther 23:403–416
Sebel PS, Lowdon JD (1989) Propofol: a new intravenous anesthetic. Anesthesiology 71:260–277
Simons PJ, Cockshott ID, Douglas EJ, Gordon EA, Hopkins K, Rowland M (1988) Disposition in male volunteers of a subanaesthetic intravenous dose of an oil in water emulsion of 14C-propofol. Xenobiotica 18:429–440
Sneyd JR, Simons PJ, Wright B (1994) Use of proton nmr spectroscopy to measure propofol metabolites in the urine of the female Caucasian patient. Xenobiotica 24:1021–1028
Guitton J, Buronfosse T, Desage M, Flinois JP, Perdrix JP, Brazier JL, Beaune P (1998) Possible involvement of multiple human cytochrome P450 isoforms in the liver metabolism of propofol. Br J Anaesth 80:788–795
Court MH, Duan SX, Hesse LM, Venkatakrishnan K, Greenblatt DJ (2001) Cytochrome P-450 2B6 is responsible for interindividual variability of propofol hydroxylation by human liver microsomes. Anesthesiology 94:110–119
Stresser DM, Kupfer D (1999) Monospecific antipeptide antibody to cytochrome P-450 2B6. Drug Metab Dispos 27:517–525
Baker MT, Chadam MV, Ronnenberg WC Jr (1993) Inhibitory effects of propofol on cytochrome P450 activities in rat hepatic microsomes. Anesth Analg 76:817–821
Kharasch ED, Thummel KE (1993) Human alfentanil metabolism by cytochrome P450 3A3/4. An explanation for the interindividual variability in alfentanil clearance? Anesth Analg 76:1033–1039
Labroo RB, Thummel KE, Kunze KL, Podoll T, Trager WF, Kharasch ED (1995) Catalytic role of cytochrome P4503A4 in multiple pathways of alfentanil metabolism. Drug Metab Dispos 23:490–496
Tateishi T, Krivoruk Y, Ueng YF, Wood AJ, Guengerich FP, Wood M (1996) Identification of human liver cytochrome P-450 3A4 as the enzyme responsible for fentanyl and sufentanil N-dealkylation. Anesth Analg 82:167–172
Kronbach T, Mathys D, Umeno M, Gonzalez FJ, Meyer UA (1989) Oxidation of midazolam and triazolam by human liver cytochrome P450IIIA4. Mol Pharmacol 36: 89–96
Leung BP, Miller E, Park GR (1997) The effect of propofol on midazolam metabolism in human liver microsome suspension. Anaesthesia 52:945–948
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanaka, E., Takano, Y., Inomata, S. et al. Premedication medicines do not cause drug metabolic interaction with propofol using human liver microsomes in vitro. Eur J Clin Pharmacol 60, 565–568 (2004). https://doi.org/10.1007/s00228-004-0807-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-004-0807-3