Abstract
Objective
A prospective population pharmacokinetic study of nevirapine (NVP) was performed to test the relationship between hepatotoxicity and NVP trough plasma concentration and to identify which covariates could influence NVP pharmacokinetics.
Methods
All patients [77 HIV-1 (human immunodeficiency virus type 1)-infected patients (128 samples)] were either on first-line antiretroviral therapy or switched from successful therapy containing protease inhibitor. Population pharmacokinetic parameters were estimated by a non-linear mixed-effect modelling method. Hepatotoxicity was evaluated by ASAT (aspartate aminotransferase) plasma level.
Results
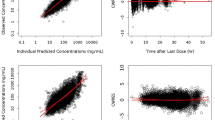
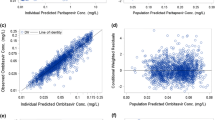
No correlation was found between high NVP trough plasma concentration and high ASAT level or the increase of ASAT level on NVP therapy. Age and Caucasian race were found to be significant covariates of NVP clearance (Cl/F). Population pharmacokinetic parameters (rate absorption constant=1.04 h−1; Cl/F=3.31 h−1; apparent volume of distribution=92 l) are consistent with previous studies.
Conclusion
High NVP trough plasma concentrations are not correlated with hepatotoxicity in our population. NVP clearance is decreased in the elderly patients, suggesting a potential increase of NVP plasma level and the interest of therapeutic drug monitoring for this population.




Similar content being viewed by others
References
Aarnoutse RE, Schapiro JM, Boucher, CAB, Hekster YA, Burger DM (2003) Therapeutic drug monitoring: an aid to optimising response to antiretroviral drugs? Drugs 63:741–753
Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T (2001) Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS 15:71–75
Veldkamp AI, Weverling GJ, Lange JM, Montaner JS, Reiss P, Copper DA, Vella S, Hall D, Beijnen JH, Hoetelmans RM (2001) High exposure to nevirapine in plasma is associated with an improved virological response in HIV-1-infected individuals. AIDS 15:1089–1095
Gonzalez de Requena D, Nunez M, Jimenez-Nacher I, Soriano V (2002) Liver toxicity caused by nevirapine. AIDS 16:290–291
De Maat MMR, Mathot RAA, Veldkamp AI, Huitema ADR, Mulder JW, Meenhorst PL, Van Gorp ECM, Carlier H, Beijnen JH (2002) Hepatotoxicity following nevirapine-containing regimens in HIV-1-infected individuals. Pharmacol Res 46:295–300
Dailly E, Thomas L, Kergueris MF, Jolliet P, Bourin M (2001) High performance liquid chromatographic assay to determine the plasma levels of HIV-protease inhibitors (amprenavir, indinavir, nelfinavir, ritonavir and saquinavir) and the non-nucleoside reverse transcriptase inhibitor (nevirapine) after liquid-liquid extraction. J Chromatogr B Biomed Sci Appl 758:129–135
Mouly S, Aymard G, Tillement JP, Caulin C, Bergmann JF, Urien S (2001) Increased oral ganciclovir bioavaibility in HIV-infected patients with chronic diarrhoea and wasting syndrome—a population pharmacokinetic study. Br J Clin Pharmacol 51:557–565
De Maat MMR, Huitema ADR, Mulder JW, Meenorst PL, Van Gorp ECM, Beijnen JH (2002) Population pharmacokinetics of nevirapine in an unselected cohort of HIV-1-infected individuals. Br J Clin Pharmacol 54:378–385
Zhou XJ, Sheiner LB, D’Aquila RT, Hughes MD, Hirsch MS, Fischl MA, Johnson VA, Myers M, Sommadossi JP, The National Institute of Allergy and Infectious Diseases AIDS Clinical Trials Group Protocol 241 Investigators (1999) Population pharmacokinetics of nevirapine, zidovudine, and didanosine in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother 43:121–128
Havlir D, Cheeseman SH, McLaughlin M, Murphy R, Erice A, Spector SA, Greenough TC, Sullivan JL, Hall D, Myers M, Lamson M, Richman DD (1995) High-dose nevirapine: safety, pharmacokinetics, and antiviral effect in patients with human immunodeficiency virus infection. J Infect Dis 171:537–545
Cheeseman SH, Hattox SE, McLaughlin MM, Koup RA, Andrews C, Bova CA, Pav JW, Roy T, Sullivan JL, Keirns JJ (1993) Pharmacokinetics of nevirapine: initial single-rinsing-dose study in humans. Antimicrob Agents Chemother 37:178–182
Nunez M, Gonzalez-Requena D, Gonzalez-Lahoz J, Soriano V (2003) Interactions between nevirapine plasma levels, chronic hepatitis C, and the development of liver toxicity in HIV-infected patients. AIDS Res Hum Retroviruses 19:187–188
Acknowledgement
The authors thank Boehringer Ingelheim Pharmaceuticals Inc for their support in providing nevirapine.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dailly, E., Billaud, E., Reliquet, V. et al. No relationship between high nevirapine plasma concentration and hepatotoxicity in HIV-1-infected patients naive of antiretroviral treatment or switched from protease inhibitors. Eur J Clin Pharmacol 60, 343–348 (2004). https://doi.org/10.1007/s00228-004-0769-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-004-0769-5