Abstract
Aims
Fibrate treatment induces adverse changes in biliary-lipid and bile-acid composition. Since the molecular mechanisms underlying these changes are still unclear, we have investigated the effect of fibrate treatment on key factors involved in bile-acid synthesis, biliary-lipid secretion and cholesterol metabolism in gallstone patients.
Methods
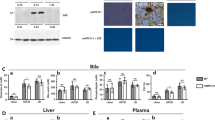
Patients with uncomplicated gallstones and a serum level of low-density lipoprotein (LDL) cholesterol >130 mg/dl were randomly assigned to open-label treatment with bezafibrate, fenofibrate, gemfibrozil, or placebo for 8 weeks before elective cholecystectomy. A liver specimen was obtained at operation, and the mRNA relative levels for cholesterol 7α-hydroxylase (CYP7A1), hepatocyte nuclear factor-4 (HNF-4), ATP-binding cassette transporters MDR3, ABCG5, and ABCG8, human homologue scavenger receptor BI, sterol response element binding protein-2 (SREBP-2), 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase and LDL receptor were determined by means of reverse-transcriptase polymerase chain reaction.
Results
Bezafibrate, fenofibrate and gemfibrozil significantly reduced CYP7A1 mRNA levels. The three fibrates tested raised the mRNA levels of ABCG5 and SREBP-2, but only bezafibrate induced significant changes. Although MDR-3 mRNA levels were slightly increased by the three fibrates, no significant differences were obtained.
Conclusions
These results show for the first time that fibrate administration to humans downregulates CYP7A1. Although ABCG5 and SREBP-2 mRNA levels were slightly increased by all treatment groups, only bezafibrate induced significant changes.




Similar content being viewed by others
References
Barbier O, Torra IP, Duguay Y, Blanquart C, Fruchart JC, Glineur C, Staels B (2002) Pleiotropic actions of peroxisome proliferator-activated receptors in lipid metabolism and atherosclerosis. Arterioscler Thromb Vasc Biol 22:717–726
Kesäniemi A, Grundy SM (1984) Influence of gemfibrozil and clofibrate on metabolism of cholesterol and plasma triglycerides in man. JAMA 251:2241–2246
Einarsson K, Angelin B (1986) Hyperlipoproteinemia, hypolipidemic treatment and gallstone disease. Atheroscler Rev 15:67–97
Stahlberg D, Reihnér E, Rudling M, Berglund M, Einarsson K, Angelin B (1995) Influence of bezafibrate on hepatic cholesterol metabolism in gallstone patients: reduced activity of cholesterol 7alpha-hydroxylase. Hepatology 21:1025–1030
Post SM, Duez H, Gervois PP, Kuipers F, Princen HMG (2001) Fibrates suppress bile acid synthesis via peroxisome proliferator-activated receptor-alpha-mediated downregulation of cholesterol 7 alpha-hydroxylase and sterol 27-hydroxylase expression. Arterioscler Thromb Vasc Biol 21:1840–1845
Bertolotti M, Concari M, Loria P, Abate N, Pinetti A, Guicciardi ME, Carulli N (1995) Effects of different phenotypes of hypercholesterolemia and of treatment with fibric acid derivatives on the rates of cholesterol 7alpha hydroxylation in humans. Arterioscler Thromb Vasc Biol 15:1064–1069
Princen HMG, Post SM, Twisk J (1997) Regulation of bile acid biosynthesis. Curr Pharm Des 3:59–84
Chiang JYL (1998) Regulation of bile acid synthesis. Front Biosci 3:D176–D193
Davis RA, Miyake JH, Hui TY, Spann NJ (2002) Regulation of cholesterol-7alpha-hydroxylase: barely missing a SHP. J Lipid Res 43:533–543
Russell DW, Setchell KD (1992) Bile acid byosinthesis. Biochemistry 31:4737–4749
Wang DPD, Stroup M, Marrapodi M, Crestani M, Galli G, Chiang JYL (1996) Transcriptional regulation of the human cholesterol 7 alpha-hydroxylase gene (CYP7A9) in HepG2 cells. J Lipid Res 37:1831–1841
Tobin KA, Steineger HH, Alberti S, Spydevol O, Auwerx J, Gustafsson JK, Nebb HI (2000) Cross-talk between fatty acid and cholesterol metabolism mediated by liver X receptor alpha. Mol Endocrinol 14:741–752
Chiang JY, Kimmel R, Stroup D (2001) Regulation of cholesterol 7alpha-hydroxylase gene (CYP7A1) transcription by the liver orphan receptor (LXRalpha). Gene 262:257–265
Bocos C, Castro M, Quack G, Herrera E (1993) Studies with etofibrate in the rat. II: a comparison of the effects of prolonged and acute administration on plasma lipids, liver enzymes and adipose tissue lipolysys. Biochim Biophys Acta 1168:340–347
Combettes-Souverain M, Milliat F, Sérougne C, Férézou J, Lutton C (1999) SR-BI et metabolisme du cholesterol. Medicine/Sciences 15:1252–1258
Sehayek E, Ono JG, Shefer S, Nguyen LB, Wang N, Batta AK, Salen G, Smith JD, Tall AR, Breslow JL (1998) Biliary cholesterol excretion: a novel mechanism that regulates cholesterol absorption. Proc Natl Acad Sci U S A 95:10194–10199
Fucks M, Ivandic B, Müller O, Schalla C, Scheibner J, Bartsch P, Stange EF (2001) Biliary cholesterol hypersecretion in gallstone-susceptible mice is associated with hepatic up-regulation of the high-density lipoprotein receptor SRBI. Hepatology 33:1451–1459
Mardones P, Quiñone V, Amigo L, Moreno M, Miquel JF, Schwarz M, Miettinen HE, Trigatti B, Krieger M, VanPatten S, Cohen DE, Rigotti A (2001) Hepatic cholesterol and bile acid metabolism and intestinal cholesterol absorption in scavenger receptor class B type I-deficient mice. J Lipid Res 42:170–180
Schmitz G, Langmann T, Heimerl S (2001) Role of ABCG1 and other ABCG family members in lipid metabolism. J Lipid Res 42:1513–1520
Borst P, Zelcer N, van Helvoort A (2000) ABC transporters in lipid transport. Biochim Biophys Acta 1486:128–144
Chinetti G, Gbaguidi FG, Griglio S, Mallat Z, Antonucci M, Poulain P, Chapmam J, Fruchart JC, Tedgui A, Najib-Fruchart J, Staels B (2000) CLA-1/SR-BI expressed in atherosclerotic lesion macrophages and regulated by activators of peroxisome proliferator-activated receptors. Circulation 101:2411–2417
Chianale J, Vollrath V, Wielandt AM, Amigo L, Rigotti A, Nervi F, Gonzalez S, Andrade L, Pizarro M, Accatino L (1996) Fibrates induce mdr2 gene expression and biliary phospholipid secretion in the mouse. Biochem J 314:781–786
Miranda S, Vollrath V, Wielandt AM, Loyola G, Bronfman M, Chianale J (1997) Overexpression of mdr2 gene by peroxisome proliferators in the mouse liver. J Hepatol 26:1331–1339
Wang J, Freeman DJ, Grundy SM, Levine DM, Guerra R, Cohen JC (1998) Linkage between cholesterol 7 alpha-hydroxylase and high plasma low-density lipoprotein cholesterol concentrations. J Clin Invest 101:1283–1291
Pullinger CR, Eng C, Salen G, Shefer S, Batta AK, Erickson SK, Verhagen A, Rivera CR, Mulvihill SJ, Malloy MJ, Kane JP (2002) Human cholesterol 7 α-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J Clin Invest 110:109–117
Roglans N, Peris C, Verd JC, Alegret M, Vázquez M, Sánchez RM, Laguna JC (2001) Increase in hepatic expression of SREBP-2 by gemfibrozil administration to rats. Biochem Pharmacol 62:803–809
Brown MS, Goldstein JL (1997) The SREBP pathway: regulation of cholesterol metabolism by proteolisis of a membrane-bound transcription factor. Cell 89:331–340
Horton JD, Shimoura I (1999) Sterol regulatory element-binding proteins activators of cholesterol and fatty acid biosynthesis. Curr Opin Lipidol 10:143–150
Roglans N, Bellido A, Rodríguez C, Cabrero A, Novell F, Ros E, Zambón D, Laguna JC (2002) Fibrate treatment does not modify the expression of acyl-CoA oxidase in human liver. Clin Pharmacol Ther 72:692–701
Roglans N, Sanguino E, Peris C, Alegret M, Vázquez M, Adzet T, Díaz C, Hernández G, Laguna JC, Sánchez RM (2002) Atorvastatin treatment induced PPAR alpha expression and decreased plasma non-esterified fatty acids and liver triglyceride in fructose fed rats. J Pharmacol Exp Ther 302:232–239
Gause WC, Adamovicz J (1995) Use of PCR to quantitate relative differences in gene expression. In: Dieffenbach CW and Dveksler GS (eds) PCR primer. A laboratory manual. Cold Spring Harbor Laboratory Press, New York
Cheema SK, Agellon LB (2000) The murine and human cholesterol 7 alpha hydroxylase gene promoters are differentially responsive to regulation by fatty acids mediated via peroxisome proliferator-activated receptor alpha. J Biol Chem 275:12530–12536
Marrapodi M, Chiang JYL (2000) Peroxisome proliferator-activated receptor alpha (PPARalpha) and agonist inhibit cholesterol 7 alpha hydroxylase gene (CYP7A1) transcription. J Lipid Res 41:514–520
Patel DD, Knight BL, Soutar AK, Gibbons GF, Wade DP (2000) The effect of peroxisome-proliferator-activated receptor alpha on the activity of the cholesterol 7 alpha hydroxylase gene. Biochem J 351:747–753
Stahlberg D, Reihnér E, Ewerth S, Einarsson K, Angelin B (1991) Effects of bezafibrate on hepatic cholesterol metabolism. Eur J Clin Pharmacol 40:S33–S36
Yu L, Hammer RE, Li-Hawkins J, Von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH (2002) Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc Natl Acad Sci U S A 99:16237–16242
Yu L, Li-Hawkins J, Hammer RE, Berge KE, Horton JD, Cohen JC, Hobbs HH (2002) Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J Clin Invest 110:671–680
Einarsson K, Angelin B (1986) Hyperlipoproteinemia, hypolipidemic treatment, and gallstone disease. Atheroscler Rev 15:67–97
Sato R, Inoue J, Kawabe Y, Kodama T, Takano T, Maeda M (1996) Sterol-dependent transcriptional regulation of sterol regulatory element-binding protein-2. J Biol Chem 271:26461–26464
Acknowledgements
The study was financially supported by grants from the Fundació Privada Catalana de Nutrició i Lípids, Comisión Interministerial de Ciencia y Tecnologia (SAF98–0105; SAF00–0201), MCYT (BFI 2002–05167) and Generalitat de Catalunya (2001SGR00141).
Author information
Authors and Affiliations
Corresponding author
Additional information
E.R., D.Z., and J.C.L. are members of Institut d’Investigació Biomèdica August Pi i Sunyer (IDIBAPS).
Rights and permissions
About this article
Cite this article
Roglans, N., Vázquez-Carrera, M., Alegret, M. et al. Fibrates modify the expression of key factors involved in bile-acid synthesis and biliary-lipid secretion in gallstone patients. Eur J Clin Pharmacol 59, 855–861 (2004). https://doi.org/10.1007/s00228-003-0704-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-003-0704-1