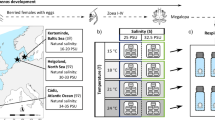
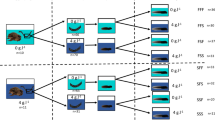
Abstract
Understanding organismal responses to environmental drivers is relevant to predict species capacities to respond to climate change. However, the scarce information available on intraspecific variation in the responses oversimplifies our view of the actual species capacities. We studied intraspecific variation in survival and larval development of a marine coastal invertebrate (shore crab Carcinus maenas) in response to two key environmental drivers (temperature and salinity) characterising coastal habitats. On average, survival of early larval stages (up to zoea IV) exhibited an antagonistic response by which negative effects of low salinity were mitigated at increased temperatures. Such response would be adaptive for species inhabiting coastal regions of freshwater influence under summer conditions and moderate warming. Average responses of developmental time were also antagonistic and may be categorised as a form of thermal mitigation of osmotic stress. The capacity for thermal mitigation of low-salinity stress varied among larvae produced by different females. For survival in particular, deviations did not only consist of variations in the magnitude of the mitigation effect; instead, the range of responses varied from strong effects to no effects of salinity across the thermal range tested. Quantifying intraspecific variation of such capacity is a critical step in understanding responses to climate change: it points towards either an important potential for selection or a critical role of environmental change, operating in the parental environment and leading to stress responses in larvae.




Similar content being viewed by others
References
Anger K (1991) Effects of temperature and salinity on the larval development of the Chinese mitten crab Eriocheir sinensis (Decapoda: Grapsidae). Mar Ecol Prog Ser 72:103–110
Anger K, Spivak E, Luppi T (1998) Effects of reduced salinities on development and bioenergetics of early larval shore crab, Carcinus maenas. J Exp Mar Biol Ecol 220:287–304
Appelbaum SL, Pan TCF, Hedgecock D, Manahan DT (2014) Separating the nature and nurture of the allocation of energy in response to global change. Integr Comp Biol 54:284–295
Bils F, Kanstinger P, Kloppmann MHF, Peck MA (2012) Habitat partitioning by fish larvae among coastal, offshore and frontal zones in the southern North Sea. Aquat Biol 15:237–250
Bolnick DI, Amarasekare P, Araújo MS, Bürger R, Levine JM, Novak M, Rudolf VHW, Schreiber SJ, Urban MC, Vasseur D (2011) Why intraspecific trait variation matters in community ecology. Trends Ecol Evol 26(4):183–192
Boyd PW, Collins S, Dupont S, Fabricius K, Gattuso J-P, Havenhand J, Hutchins DA, Riebesell U, Rintoul MS, Vichi M, Biswas H, Ciotti A, Gao K, Gehlen M, Hurd CL, Kurihara H, McGraw CM, Navarro JM, Nilsson GE, Passow U, Pörtner H-O (2018) Experimental strategies to assess the biological ramifications of multiple drivers of global ocean change—a review. Glob Change Biol 24:2239–2261
Brierley AS, Kingsford MJ (2009) Impacts of climate change on marine organisms and ecosystems. Curr Biol 19:R602–R614
Campbell PJ, Jones MB (1989) Osmoregulation of the estuarine prawn Palaemon longirostris (Caridea: Palaemonidae). J Mar Biol Assoc UK 69:261–272
Carter HA, Ceballos-Osuna L, Miller NA, Stillman JH (2013) Impact of ocean acidification on metabolism and energetics during early life stages of the intertidal porcelain crab Petrolisthes cinctipes. J Exp Biol 216:1412–1422
Charmantier G (1998) Ontogeny of osmoregulation in crustaceans: a review. Invertebr Reprod Dev 33(2–3):177–190
Christidis N, Jones GS, Stott PA (2015) Dramatically increasing chance of extremely hot summers since the 2003 European heatwave. Nature Clim Change 5:46–50
Cieluch U, Anger K, Aujoulat F, Buchholz F, Charmantier-Daures M, Charmantier G (2004) Ontogeny of osmoregulatory structures and functions in the green crab Carcinus maenas (Crustacea, Decapoda). J Exp Biol 207:325–336
Compton TJ, Leathwick JR, Inglis GJ (2010) Thermogeography predicts the potential global range of the invasive European green crab (Carcinus maenas). Divers Distrib 16:243–255
Côté IM, Darling ES, Brown CJ (2016) Interactions among ecosystem stressors and their importance in conservation. Proc R Soc Lond B 283:2015–2592
Cowen RK, Sponaugle S (2009) Larval dispersal and marine population connectivity. Annu Rev Mar Sci 1:443–466
Cowgill UM, Williams DM, Esquivel JB (1984) Effects of maternal nutrition on fat-content and longevity of neonates of Daphnia magna. J Crustacean Biol 4:173–190
Crain CM, Kroeker K, Halpern BS (2008) Interactive and cumulative effects of multiple human stressors in marine systems. Ecol Lett 11:1304–1315
Dawirs RR (1985) Temperature and larval development of Carcinus maenas (Decapoda) in the laboratory; predictions of larval dynamics in the sea. Mar Ecol Prog Ser 24:297–302
De Laender F (2018) Community-and ecosystem- level effects of multiple environmental change drivers: beyond null model testing. Glob Change Biol 24:5021–5030
Denny M (2017) The fallacy of the average: on the ubiquity, utility and continuing novelty of Jensen’s inequality. J Exp Biol 220:139–146
deRivera CE, Hitchcock NG, Teck SJ, Stevens BP, Hines AH, Ruiz GM (2007) Larval development rate predicts range expansion of an introduced crab. Mar Biol 150:1275–1288
Domingues CP, Creer S, Taylor MI, Queiroga H, Carvalho GR (2010) Genetic structure of Carcinus maenas within its native range: larval dispersal and oceanographic variability. Mar Ecol Prog Ser 410:111–123
Donelson JM, Munday PL, McCormick MI, Nilsson GE (2011) Acclimation to predicted ocean warming through developmental plasticity in a tropical reef fish. Glob Change Biol 17:1712–1719
Doney SC, Ruckelshaus M, Duffy JE, Barry JP, Chan F, English CA, Galindo HM, Grebmeier JM, Hollowed AB, Knowlton N, Polovina J, Rabalais NN, Sydeman WJ, Talley LD (2012) Climate change impacts on marine ecosystems. Annu Rev Mar Sci 4:11–37
Durrant HMS, Clark GF, Dworjanyn SA, Byrne M, Johnston EL (2013) Seasonal variation in the effects of ocean warming and acidification on a native bryozoan, Celleporaria nodulosa. Mar Biol 160:1903–1911
Flügel H (1963) Elektrolytregulation und Temperatur bei Crangon crangon L. und Carcinus maenas L. Kiel Meeresforsch 19:189–195
Folt CL, Chen CY, Moore MV, Burnaford J (1999) Synergism and antagonism among multiple stressors. Limnol Oceanogr 44:864–877
Fregly MJ (2011) Adaptations: some general characteristics. Comp Physiol 14:3–15
Galecki A, Burzykowski T (2013) Linear Mixed-Effect Models Using R. Springer, New York, pp 245–273
Gattuso JP, Hansson L (2009) Ocean Acidification. Oxford University Press, Oxford
Giménez L, Anger K (2001) Relationships among salinity, egg size, embryonic development, and larval biomass in the estuarine crab Chasmagnathus granulata Dana, 1851. J Exp Mar Biol Ecol 260:241–257
Giménez L, Anger K (2003) Larval performance in an estuarine crab, Chasmagnathus granulata, is a consequence of both larval and embryonic experience. Mar Ecol Prog Ser 249:251–264
González-Ortegón E, Giménez L (2014) Environmentally mediated phenotypic links and performance in larvae of a marine invertebrate. Mar Ecol Prog Ser 502:185–195
Gunderson AR, Armstrong EJ, Stillman JH (2016) Multiple stressors in a changing world: the need for an improved perspective on physiological responses to the dynamic marine environment. Annu Rev Mar Sci 8:12.1–12.22
Harvey BP, Gwynn-Jones D, Moore PJ (2013) Meta-analysis reveals complex marine biological responses to the interactive effects of ocean acidification and warming. Ecol Evol 3(4):1016–1030
Hoegh-Guldberg O, Bruno JF (2010) The impact of climate change on the world‘s marine ecosystems. Science 328:1523–1528
IPCC (2014) Climate change 2014: synthesis report. In: Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change [Core writing team, Pachauri RK, Meyer LA (eds)] IPCC, Geneva, Switzerland, p 151
Janas U, Spicer J (2008) Does the effect of low temperature on osmoregulation by the prawn Palaemon elegans Rathke, 1837 explain winter migration offshore? Mar Biol 153(5):937–943
Kindsvater HK, Otto SP (2014) The evolution of offspring size across life-history stages. Am Nat 184:543–555
Kinne O (1971) Salinity. Marine ecology. A comprehensive, integrated treatise on life in oceans and coastal waters. Wiley, London
Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo L, Singh GS, Duarte CM, Gattuso J-P (2013) Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob Change Biol 19:1884–1896
Lange R, Marshall D (2017) Ecologically relevant levels of multiple, common marine stressors suggest antagonistic effects. Sci Rep 7(1):6281
Laughlin RB, French W (1989) Interactions between temperature and salinity during brooding on subsequent zoeal development of the mud crab Rhithropanopeus harrisii. Mar Biol 102:377–386
Lucu C, Towle DW (2003) Na+–K+–ATPase in gills of aquatic crustacea. Comp Biochem Physiol A Physiol 135:195–214
Marshall DJ, Allen RM, Crean AJ (2008) The ecological and evolutionary importance of maternal effects in the sea. Oceanogr Mar Biol Annu Rev 46:203–262
Meyer EMI, Pohlmann T, Weisse R (2011) Thermodynamic variability and change in the North Sea (1948–2007) derived from a multidecadal hindcast. J Mar Syst 86:35–44
Moksnes P-O, Corell H, Tryman K, Hordoir R, Jonsson PR (2014) Larval behavior and dispersal mechanisms in shore crab larvae (Carcinus maenas): local adaptations to different tidal environments? Limnol Oceanogr 59:588–602
Nagaraj M (1993) Combined effects of temperature and salinity on the zoeal development of the green crab, Carcinus maenas (Linnaeus, 1758) (Decapoda, Portunidae). Sci Mar 57(1):1–8
Nasrolahi A, Pansch C, Lenz M, Wahl M (2012) Being young in a changing world: how temperature and salinity changes interactively modify the performance of larval stages of the barnacle Amphibalanus improvisus. Mar Biol 159(2):331–340
O’Connor MI, Bruno JF, Gaines SD, Halpern BS, Lester SE, Kinlan BP, Weiss JM (2007) Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation. Proc Natl Acad Sci 104:1266–1271
Palumbi SR (2003) Population genetics, demographic connectivity, and the design of marine reserves. Ecol Appl 13(1):S146–S158
Pandori LLM, Sorte CJB (2019) The weakest link: sensitivity to climate extremes across life stages of marine invertebrates. Oikos 128:621–629
Parker LM, O’Connor WA, Byrne M, Coleman RA, Virtue P, Dove M et al (2017) Adult exposure to ocean acidification is maladaptive for larvae of the Sydney rock oyster Saccostrea glomerata in the presence of multiple stressors. Biol Lett 13:20160798
Pechenik JA (1987) Environmental influences on larval survival and growth. In: Giese AC, Pearse JS (eds) Reproduction of marine invertebrates, vol 9. Blackwell Scientific, New York, pp 551–608
Philippart CJM, Anadón R, Danovaro R, Dippner JW, Drinkwater KF, Hawkins SJ, Oguz T, O‘Sullivan G, Reid PC (2011) Impacts of climate change on European marine ecosystems: observations, expectations and indicators. J Exp Mar Biol Ecol 400:52–69
Piggott JJ, Townsend CR, Matthaei CD (2015) Reconceptualizing synergism and antagonism among multiple stressors. Ecol Evol 5(7):1538–1547
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2018). nlme: linear and nonlinear mixed effects models. R package version 3.1–137. https://CRAN.R-project.org/package=nlme. Accessed 11 Dec 2018
Pond D, Harris R, Head R, Harbour D (1996) Environmental and nutritional factors determining seasonal variability in the fecundity and egg viability of Calanus helgolandicus in coastal waters off Plymouth, UK. Mar Ecol Progr Ser 143:45–63
Pörtner HO (2010) Oxygen– and capacity–limitation of thermal tolerance: a matrix for integrating climate–related stressor effects in marine ecosystems. J Exp Biol 213:881–893
Przeslawski R, Byrne M, Mellin C (2015) A review and meta–analysis of the effects of multiple abiotic stressors on marine embryos and larvae. Glob Change Biol 21(6):2122–2140
Robins PE, Skov MW, Lewis MJ, Giménez L, Davies AG, Malham SK, Neill SP, McDonald JE, Whitton TA, Jackson SE, Jago CF (2015) Impact of climate change on UK estuaries: a review of past trends and potential projections. Estuar Coast Shelf Sci 169:119–135
Roman J, Palumbi SR (2004) A global invader at home: population structure of the green crab, Carcinus maenas, in Europe. Mol Ecol 13:2891–2898
RStudio Team (2016) RStudio: integrated development for R. RStudio, Inc., Boston, MA. http://www.rstudio.com/
Schäfer RB, Piggott JJ (2018) Advancing understanding and prediction in multiple stressor research through a mechanistic basis for null models. Glob Change Biol 24(5):1817–1826
Schindler DE, Armstrong JB, Reed TE (2015) The portfolio concept in ecology and evolution. Front Ecol Env 13:257–263
Schrum C, Lowe J, Meier HEM, Grabemann I, Holt J, Mathis M, Pohlmann T, Skogen MD, Sterl A, Wakelin S (2016) Projected Change—North Sea. In: Quante M, Colijn F (eds) North Sea region climate change assessment. Regional climate studies. Springer Open, Berlin, pp 175–217
Shama LNS, Strobel A, Mark FC, Wegner KM (2014) Transgenerational plasticity in marine sticklebacks: maternal effects mediate impacts of a warming ocean. Funct Ecol 28:1482–1493
Sokolova IM, Frederich M, Bagwe R, Lannig G, Sukhotin AA (2012) Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar Env Res 79:1–15
Spitzner F, Meth R, Krüger C, Nischik E, Eiler S, Sombke A, Torres G, Harzsch S (2018) An atlas of larval organogenesis in the European shore crab Carcinus maenas L. (Decapoda, Brachyura, Portunidae). Front Zool 15:27
Torres G, Giménez L, Pettersen AK, Bue M, Burrows MT, Jenkins SR (2016) Persistent and context-dependent effects of the larval feeding environment on post-metamorphic performance through the adult stage. Mar Ecol Prog Ser 545:147–160
Torres G, Spitzner F, Harzsch S, Giménez L (2019) Ecological developmental biology and global ocean change: brachyuran crustacean larvae as models. In: Fusco G (ed) Perspectives in evolutionary and developmental biology. Padova University Press, Padova
Uller T, Nakagawa S, English S (2013) Weak evidence for anticipatory parental effects in plants and animals. J Evol Biol 26:2161–2170
Wiltshire KH, Kraberg A, Bartsch I, Boersma M, Franke H-D, Freund J, Gebür C, Gerdts G, Stockmann K, Wichels A (2010) Helgoland roads, North Sea: 45 years of change. Estuaries Coasts 33:295–310
Zuur A, Ieno E, Walker N, Savaliev A, Smith G (2009) Mixed effect models and extensions in ecology with R. Springer, New York
Acknowledgements
We are grateful to Julia Brinkmann, Stefan Eiler, Wladimir Escalante Alvarado, Michael Exton and Simon Wolf for their assistance in animal husbandry. We thank the students of the “Schülerlabor” (Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Helgoland, Germany) for their help in collecting the berried females at the Helgoland intertidal. We acknowledge A. Sombke for conceptual discussion and assistance during the initial phase of this project.
Funding
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, Research Training Group 2010: RESPONSE programme, work-package B3) and involved a collaboration between the working groups of S. Harzsch (Greifswald University, Germany), G. Torres (AWI-Helgoland, Germany) and L. Giménez (Bangor University, UK and AWI-Helgoland, Germany). The funding body did not influence the design of the study and collection, analysis, and interpretation of data nor the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
LG, SH and GT conceived the experiments. FS, RM and GT performed the experiments. FS and LG analysed the data. FS wrote the first draft as part of her doctoral dissertation. LG and GT wrote the final manuscript. All the authors improved the final manuscript. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interests.
Ethical standards
The research presented in this paper complies with the guidelines from the directives 2010/63/EU of the European parliament and of the Council of 22nd September 2010 on the protection of animals used for scientific purposes.
Additional information
Responsible Editor: A.E. Todgham.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by Undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Spitzner, F., Giménez, L., Meth, R. et al. Unmasking intraspecific variation in offspring responses to multiple environmental drivers. Mar Biol 166, 112 (2019). https://doi.org/10.1007/s00227-019-3560-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-019-3560-y