Abstract
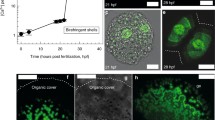
Ocean acidification (OA), the reduction of ocean pH due to hydration of atmospheric CO2, is known to affect growth and survival of marine invertebrate larvae. Survival and transport of vulnerable planktonic larval stages play important roles in determining population dynamics and community structures in coastal ecosystems. Here, we show that larvae of the purple urchin, Strongylocentrotus purpuratus, underwent high-frequency budding (release of blastula-like particles) when exposed to elevated pCO2 level (>700 μatm). Budding was observed in >50 % of the population and was synchronized over short periods of time (~24 h), suggesting this phenomenon may be previously overlooked. Although budding can be a mechanism through which larval echinoids asexually reproduce, here, the released buds did not develop into viable clones. OA-induced budding and the associated reduction in larval size suggest new hypotheses regarding physiological and ecological tradeoffs between short-term benefits (e.g. metabolic savings and predation escape) and long-term costs (e.g. tissue loss and delayed development) in the face of climate change.



Similar content being viewed by others
References
Byrne M (2011) Impact of ocean warming and ocean acidification on marine invertebrate life history stages: vulnerabilities and potential for persistence in a changing ocean. Oceanogr Mar Biol Ann Rev 49:1–42
Caldeira K, Wickett ME (2003) Anthropogenic carbon and ocean pH. Nature 425(6956):365
Chan KYK, Grünbaum D, O’Donnell MJ (2011) Effects of ocean-acidification-induced morphological changes on larval swimming and feeding. J Exp Biol 214(22):3857–3867
Cowen RK, Sponaugle S (2009) Larval dispersal and marine population connectivity. Annu Rev Mar Sci 1:443–466
Dai M, Lu Z, Zhai W, Chen B, Cao Z, Zhou K, Cai WJ, Chen CTA (2009) Diurnal variations of surface seawater pCO2 in contrasting coastal environments. Limnol Oceanogr 54(3):735–745
Dickson A, Millero F (1987) A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res (I Oceanogr Res Pap) 34(10):1733–1743
Dickson AG, Sabine CL, Christian JR (2007) Guide to best practices for ocean CO2 measurements. PICES special publication 3
Doney SC, Fabry VJ, Feely RA, Kleypas JA (2009) Ocean acidification: the other CO2 problem. Annu Rev Mar Sci 1:169–192. doi:10.1146/annurev.marine.010908.163834
Dupont S, Dorey N, Thorndyke M (2010a) What meta-analysis can tell us about vulnerability of marine biodiversity to ocean acidification? Estuar Coast Shelf Sci 89(2):182–185
Dupont S, Lundve B, Thorndyke M (2010b) Near future ocean acidification increases growth rate of the lecithotrophic larvae and juveniles of the sea star Crossaster papposus. J Exp Zool B Mol Dev Evol 314(5):382–389
Dupont S, Ortega-Martínez O, Thorndyke M (2010c) Impact of near-future ocean acidification on echinoderms. Ecotoxicology 19(3):449–462
Eaves AA, Palmer AR (2003) Widespread cloning in echinoderm larvae. Nature 425(6954):146
Feely RA, Sabine CL, Hernandez-Ayon JM, Ianson D, Hales B (2008) Evidence for upwelling of corrosive” acidified” water onto the continental shelf. Science 320(5882):1490
Flores EB, Swalla BJ (2011) Effects of Wnt pathway activation on larval cloning in the sand dollar, Dendraster excentricus. Paper presented at the annual meeting, Society for Integrative and Comparative Biology Salt Lake City, UT
Grünbaum D, Strathmann RR (2003) Form, performance and trade-offs in swimming and stability of armed larvae. J Mar Res 61(5):659–691
Hart MW, Strathmann RR (1994) Functional consequences of phenotypic plasticity in echinoid larvae. Biol Bull 186:291–299
Heyward A, Negri A (2012) Turbulence, cleavage, and the naked embryo: a case for coral clones. Science 335(6072):1064
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53(282):457–481
Kremp A, Godhe A, Egardt J, Dupont S, Suikkanen S, Casabianca S, Penna A (2012) Intraspecific variability in the response of bloom-forming marine microalgae to changed climate conditions. Ecol Evol 2(6):1195–1207
Kroeker KJ, Kordas RL, Crim RN, Singh GG (2010) Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol Lett 13(11):1419–1434. doi:10.1111/j.1461-0248.2010.01518.x
Kurihara H (2008) Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates. Mar Ecol Prog Ser 373:275–284. doi:10.3354/Meps07802
McDonald KA, Vaughn D (2010) Abrupt change in food environment induces cloning in plutei of Dendraster excentricus. Biol Bull 219(1):38–49
Mehrbach C, Culberson C, Hawley J, Pytkowicz R (1973) Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol Oceanogr 18:897–907
Menden-Deuer S (2008) Spatial and temporal characteristics of plankton-rich layers in a shallow, temperate fjord. Mar Ecol Prog Ser 355:21–30
Mullin M, Sloan P, Eppley R (1966) Relationship between carbon content, cell volume, and area in phytoplankton. Limnol Oceanogr 11(2):307–311
Pearse JS (2006) Ecological role of purple sea urchins. Science 314(5801):940–941. doi:10.1126/science.1131888
Pierrot D, Lewis E, Wallace D (2006) CO2SYS MS Excel Program developed for CO2 system calculations. ORNL/CDIAC-105 carbon dioxide information analysis center, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge, TN
Reuter KIME, Lotterhos KE, Crim RN, Thompson CA, Harley CDG (2011) Elevated pCO2 increases sperm limitation and risk of polyspermy in the red sea urchin Strongylocentrotus franciscanus. Glob Change Biol 17:163–171
Rogers-Bennett L (2007) The ecology of Strongylocentrotus franciscanus and Strongylocentrotus purpuratus. In: Lawrence JM (ed) Edible sea urchins: biology and ecology, vol 37. Elsevier Science, Amsterdam, pp 393–425
Sabine CL, Feely RA, Gruber N, Key RM, Lee K, Bullister JL, Wanninkhof R, Wong C, Wallace DWR, Tilbrook B (2004) The oceanic sink for anthropogenic CO2. Science 305(5682):367
Sarazin G, Michard G, Prevot F (1999) A rapid and accurate spectroscopic method for alkalinity measurements in sea water samples. Water Res 33(1):290–294
Sodergren E, Weinstock GM, Davidson EH, Cameron RA, Gibbs RA, Angerer RC, Angerer LM, Arnone MI, Burgess DR, Burke RD (2006) The genome of the sea urchin Strongylocentrotus purpuratus. Science 314(5801):941
Strathmann MF (1987) Reproduction and development of marine invertebrates of the Northern Pacific Coast. University of Washington Press, Seattle
Stumpp M, Dupont S, Thorndyke M, Melzner F (2011a) CO2 induced acidification impacts sea urchin larval development II: gene expression patterns in pluteus larvae. Comp Biochem Physiol A: Mol Integr Physiol 160(3):320–330
Stumpp M, Wren J, Melzner F, Thorndyke M, Dupont S (2011b) CO2 induced seawater acidification impacts sea urchin larval development I: elevated metabolic rates decrease scope for growth and induce developmental delay. Comp Biochem Physiol A: Mol Integr Physiol 160(3):331–340
Sunday JM, Crim RN, Harley CDG, Hart MW (2011) Quantifying rates of evolutionary adaptation in response to ocean acidification. PLoS ONE 6(8):e22881
Todgham AE, Hofmann GE (2009) Transcriptomic response of sea urchin larvae Strongylocentrotus purpuratus to CO2-driven seawater acidification. J Exp Biol 212(16):2579
Vaughn D (2009) Predator-induced larval cloning in the sand dollar Dendraster excentricus: might mothers matter? Biol Bull 217(2):103–114
Vaughn D (2010) Why run and hide when you can divide? Evidence for larval cloning and reduced larval size as an adaptive inducible defense. Mar Biol 157(6):1301–1312
Vaughn D, Strathmann RR (2008) Predators induce cloning in echinoderm larvae. Science 319(5869):1503
Vickery MS, McClintock JB (2000) Effects of food concentration and availability on the incidence of cloning in planktotrophic larvae of the sea star Pisaster ochraceus. Biol Bull 199(3):298–304
Wootton JT, Pfister CA, Forester JD (2008) Dynamic patterns and ecological impacts of declining ocean pH in a high-resolution multi-year dataset. Proc Natl Acad Sci USA 105(48):18848
Yu PC, Matson PG, Martz TR, Hofmann GE (2011) The ocean acidification seascape and its relationship to the performance of calcifying marine invertebrates: laboratory experiments on the development of urchin larvae framed by environmentally-relevant pCO2/pH. J Exp Mar Biol Ecol 400(1–2):288–295. doi:10.1016/j.jembe.2011.02.016
Acknowledgments
We thank R. Strathmann for his helpful comments, the valuable suggestions from the reviewers, staff, and research scientists at the Sven Lovén Centre for Marine Sciences for logistic support and insightful discussions. K.C. was partially funded by a Boeing International Fellowship. D.G and K.C. were supported by Washington Sea Grant (NA10OAR4170057). SD is funded by the Linnaeus Centre for Marine Evolutionary Biology at the University of Gothenburg (http://www.cemeb.science.gu.se/) and supported by a Linnaeus grant from the Swedish Research Councils VR and Formas. This paper is a contribution to the “Sub-seabed carbon storage and the marine environment”, (ECO2) a Collaborative Project funded under the European Commission’s Framework Seven Program Topic OCEAN.2010.3, project number 265847.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H.-O. Pörtner.
Rights and permissions
About this article
Cite this article
Chan, K.Y.K., Grünbaum, D., Arnberg, M. et al. Ocean acidification induces budding in larval sea urchins. Mar Biol 160, 2129–2135 (2013). https://doi.org/10.1007/s00227-012-2103-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-012-2103-6