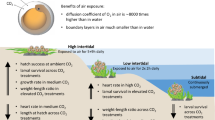
Abstract
Anthropogenic CO2 emissions are acidifying the world’s oceans. A growing body of evidence demonstrates that ocean acidification can impact survival, growth, development and physiology of marine invertebrates. Here, we tested the impact of long-term (up to 16 months) and trans-life-cycle (adult, embryo/larvae and juvenile) exposure to elevated pCO2 (1,200 μatm, compared to control 400 μatm) on the green sea urchin Strongylocentrotus droebachiensis. Female fecundity was decreased 4.5-fold when acclimated to elevated pCO2 for 4 months during reproductive conditioning, while no difference was observed in females acclimated for 16 months. Moreover, adult pre-exposure for 4 months to elevated pCO2 had a direct negative impact on subsequent larval settlement success. Five to nine times fewer offspring reached the juvenile stage in cultures using gametes collected from adults previously acclimated to high pCO2 for 4 months. However, no difference in larval survival was observed when adults were pre-exposed for 16 months to elevated pCO2. pCO2 had no direct negative impact on juvenile survival except when both larvae and juveniles were raised in elevated pCO2. These negative effects on settlement success and juvenile survival can be attributed to carry-over effects from adults to larvae and from larvae to juveniles. Our results support the contention that adult sea urchins can acclimate to moderately elevated pCO2 in a matter of a few months and that carry-over effects can exacerbate the negative impact of ocean acidification on larvae and juveniles.






Similar content being viewed by others
References
Alcorn NJ, Allen JD (2009) How do changes in parental investment influence development in echinoid echinoderms? Evol Dev 11:719–727
Bertram DF, Phillips NE, Strathmann RR (2009) Evolutionary and experimental change in egg volume, heterochrony of larval body and juvenile rudiment, and evolutionary reversibility in pluteus form. Evol Dev 11:728–739
Byrne M, Prowse TAA, Sewell MA, Dworjanyn S, Williamson JE, Vaïtilingon D (2008) Maternal provisioning for larvae and larval provisioning for juveniles in the toxopneustid sea urchin Tripneustes gratilla. Mar Biol 5:473–482
Dashfield SL, Somerfield PJ, Widdicombe S, Austen MC, Nimmo M (2008) Impacts of ocean acidification and burrowing urchins on within-sediment pH profiles and subtidal nematode communities. J Exp Mar Biol Ecol 365:46–52
Dickson AG, Millero FJ (1987) A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res 34:1733–1743
Dickson AG, Sabine CL, Christian JR (2007) Guide to best practices for ocean CO2 measurements. PICES Special Publications
Doney SC, Fabry VJ, Feely RA, Kleypas JA (2009) Ocean acidification: the other CO2 problem. Annu Rev Mar Sci 1:169–192
Dupont S, Havenhand J, Thorndyke W, Peck L, Thorndyke M (2008) Near-future level of CO2-driven ocean acidification radically affects larval survival and development in the brittlestar Ophiothrix fragilis. Mar Ecol Prog Ser 373:285–294
Dupont S, Ortega-Martínez O, Thorndyke M (2010a) Impact of near-future ocean acidification on echinoderms. Ecotoxicology 19:449–462
Dupont S, Lundve B, Thorndyke M (2010b) Near future ocean acidification increases growth rate of the lecithotrophic larvae and juveniles of the sea star Crossaster papposus. J Exp Zool B 314:382–389
Emlet RB, Hoegh-Guldberg O (1997) Effects of egg size on postlarval performance: experimental evidence from a sea urchin. Evolution 51:141–152
Emlet RB, Sadro SS (2006) Linking stages of life history: how larval quality translates into juvenile performance for an intertidal barnacle (Balanus glandula). Integr Comp Biol 46:334–346
Falk-Petersen IB, Lønning S (1983) Reproductive cycles of two closely related sea urchin species, Strongylocentrotus droebachiensis (O.F. Müller) and Strongylocentrotus pallidus (G.O. Sars). Sarsia 68:157–164
Findlay HS, Wood HL, Kendall MA, Spicer JI, Twichett RJ, Widdicombe S (2011) Comparing the impact of high CO2 on calcium carbonate structures in different marine organisms. Mar Biol Res 7:565–575
Gilbert SF, Epel D (2008) Ecological developmental biology: integrating epigenetics, medicine and evolution. Sinauer Associates Inc, Sunderland, p 480
Gooding RA, Harley CDG, Tang E (2009) Elevated water temperature and carbon dioxide concentration increase the growth of a keystone echinoderm. Proc Nat Acad Sci 106:9316–9321
Gosselin LA (1997) An ecological transition during juvenile life in a marine snail. Mar Ecol Prog Ser 157:185–194
Green MA, Jones ME, Boudreau CL, Moore RL, Westman BA (2004) Dissolution mortality of juvenile bivalves in coastal marine deposits. Limnol Oceanogr 49:727–734
Guillard RRL, Ryther JH (1962) Studies of marine planktonic diatoms. I. Cyclotella nana Husted, and Detonula confervacea (Cleve). Can J Microbiol 8:229–239
Hernández JC, Russell MP (2010) Substratum cavities affect growth-plasticity, allometry, movement and feeding rates in the sea urchin Strongylocentrotus purpuratus. J Exp Biol 213:520–525
Hernroth B, Baden S, Thorndyke M, Dupont S (2011) Immune suppression of the echinoderm Asterias rubens (L.) following long-term ocean acidification. Aquat Toxicol 103:222–224
Hoegh-Guldberg O, Emlet RB (1997) Energy use during the development of a lecithotrophic and planktotrophic echinoid. Biol Bull 192:27–40
Kålås JA, Viken Å, Bakken T. (2006). Norsk rødliste. Norwegian red list. Norway: Artsdatabanken
Kurihara H, Ishimatsu A (2008) Effects of high CO2 seawater on the copepod (Acartia tsuensis) through all life stages and subsequent generations. Mar Poll Bull 56:1086–1090
Kurihara H, Shimode S, Shirayama Y (2004) Effects of raised CO2 concentration on the egg production rate and early development of two marine copepods (Acartia steueri and Acartia erythraea). Mar Pollut Bull 49:721–727
Kurihara H, Matsui M, Furukawa H, Hayashi M, Ishimatsu A (2008) Long-term effects of predicted future seawater CO2 conditions on the survival and growth of the marine shrimp Palaemon pacificus. J Exp Mar Biol Ecol 367:41–46
Langenbuch M, Pörtner HO (2004) High sensitivity to chronically elevated CO2 levels in a eurybathic marine sipunculid. Aquat Toxicol 70: 55–61
Lau DCC, Lau SCK, Qian P-Y, Qiu J-W (2009) Morphological plasticity and resource allocation in response to food limitation and hyposalinity in a Sea Urchin. J Shellfish Res 28:383–388
Levitan DR (1991) Skeletal changes in the test and jaws of the sea urchin Diadema antillarum in response to food limitation. Mar Biol 111:431–435
Lewis E, Wallace DWR (1998) CO2SYS—program developed for the CO2 system calculations. Carbon Dioxide Inf Anal Center Report ORNL/CDIAC-105
Lucas MI, Walker G, Holland DL, Crisp DJ (1979) An energy budget for the free-swimming and metamorphosing larvae of Balanus balanoides (Crustacea: Cirripedia). Mar Biol 55:221–229
Mayor DJ, Matthews C, Cook K, Zuur AF, Hay S (2007) CO2-induced acidification affects hatching success in Calanus finmarchicus. Mar Ecol Prog Ser 350:91–97
McDonald MR, McClintock JB, Amsler CD, Rittschof D, Angus RA, Orihuela B, Lutostanski K (2009) Effects of ocean acidification over the life history of the barnacle Amphibalanus amphitrite. Mar Ecol Prog Ser 385:179–187
Mehrbach C, Culberson CH, Hawley JE, Pytkowicz RM (1973) Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol Oceanogr 18:897–907
Melzner F, Stange P, Trübenbach K, Thomsen J, Casties I, Panknin U, Gorb SN, Gutowska MA (2011) Food supply and seawater pCO2 impact calcification and internal shell dissolution in the blue mussel Mytilus edulis. PloS One 6(9):e24223. doi:10.1371/journal.pone.0024223
Mullin MM, Sloan PR, Eppley RW (1966) Relationship between carbon content, cell volume, and area in phytoplankton. Limnol Oceanogr 11:307–311
Norderhaug KM, Christie HC (2009) Sea urchin grazing and kelp re-vegetation in the NE Atlantic. Mar Biol Res 5:515–528
Parker LM, Ross OM, O’Connor WA (2011) Populations of the Sydney rock oyster, Saccostrea glomerata, vary in response to ocean acidification. Mar Biol 158:689–697
Parker LM, Ross PM, O’Connor WA, Borysko L, Raftos DA, Pörtner H-O (2012) Adult exposure influences offspring response to ocean acidification in oysters. Glob Change Biol 18:82–92
Pechenik JA (2006) Larval experience and latent effects—metamorphosis is not a new beginning. Integr Comp Biol 46:323–333
Podolsky RD, Moran AL (2006) Integrating function across marine life cycles. Integr Comp Biol 46:577–586
Russell MP (1998) Resource allocation plasticity in sea urchins: rapid, diet induced, phenotypic changes in the green sea urchin, Strongylocentrotus droebachiensis (Müller). J Exp Mar Biol Ecol 220:1–14
Sarazin G, Michard G, Prevot F (1999) A rapid and accurate spectroscopic method for alkalinity measurements in sea water samples. Water Res 33:290–294
Selden R, Johnson AS, Ellers O (2009) Waterborne cues from crabs induce thicker skeletons, smaller gonads and size-specific changes in growth rate in sea urchins. Mar Biol 156:1057–1071
Shapiro SS, Wilk MB (1965) An analysis of variance test for normality (complete samples). Biometrika 52:591–611
Shirayama Y, Thornton H (2005) Effect of increased atmospheric CO2 on shallow water marine benthos. J Geophys Res 110:C09–s08
Siikavuopio SI, Mortensen A, Dale T, Foss A (2007) Effects of carbon dioxide exposure on feed intake and gonad growth in green sea urchin, Strongylocentrotus droebachiensis. Aquacult 266:97–101
Sokal RR, Rohlf FJ (1995) Biometry. Freeman & Co, New York, p 887
Spicer JI, Widdicombe S, Needham HR, Berge JA (2011) Impact of CO2-acidified seawater on the extracellular acid-base balance of the northern sea urchin Strongylocentrotus droebachiensis. J Exp Mar Biol Ecol 407:19–25
Stumpp M, Wren J, Melzner F, Thorndyke MC, Dupont S (2011) CO2 induced seawater acidification impacts sea urchin larval development I: elevated metabolic rates decrease scope for growth and induce developmental delay. Comp Biochem Physiol A Mol Int Physiol 160:320–330
Stumpp M, Trübenbach K, Brennecke D, Hu MY, Melzner F (2012) Resource allocation and extracellular acid-base status in the sea urchin Strongylocentrotus droebachiensis in response to CO2 induced seawater acidification. Aquat Toxicol. doi:10.1016/j.aquatox.2011.12.020
Sunday JM, Crim RN, Harley CDG, Hart MW (2011) Quantifying rates of evolutionary adaptation in response to ocean acidification. PLoS ONE 6:e22881
Uthicke S, Soars N, Foo S, Byrne M (2012) Physiological effects of increased pCO2 and the effects of parent acclimation on development in the tropical Pacific sea urchin Echinometra mathaei. Marine Biology (submitted)
Vadas RL, Beal B, Dowling T, Fegley JC (2000) Experimental field tests of natural algal diets on gonad index and quality in the green sea urchin, Strongylocentrotus droebachiensis: a case for rapid summer production in post-spawned animals. Aquaculture 182:115–135
Videla JA, Chaparro OR, Thompson RJ, Concha II (1998) Role of biochemical energy reserves in the metamorphosis and early juvenile development of the oyster Ostrea chilensis. Mar Biol 132:635–640
Wood HL, Spicer JI, Widdicombe S (2008) Ocean acidification may increase calcification rates, but at a cost. Proc R Soc B 275:1767–1773
Wood HL, Spicer JI, Lowe DM, Widdicombe S (2010) Interaction of ocean acidification and temperature; the high cost of survival in the brittlestar Ophiura ophiura. Mar Biol 157:2001–2013
Acknowledgments
SD is funded by the Linnaeus Centre for Marine Evolutionary Biology at the University of Gothenburg (http://www.cemeb.science.gu.se/) and supported by a Linnaeus-grant from the Swedish Research Councils VR and Formas; VR and Formas grants to MT; Knut and Alice Wallenberg’s minnen and the Royal Swedish Academy of Sciences. FM is funded by the DFG Excellence Cluster “Future Ocean” and the German “Biological impacts of ocean acidification (BIOACID)” project 3.1.4, funded by the Federal Ministry of Education and Research (BMBF, FKZ 03F0608A). This paper is a contribution to NoAA. and the “European Project on Ocean Acidification” (EPOCA) that received funding from the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement n°211384.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. O. Pörtner.
Rights and permissions
About this article
Cite this article
Dupont, S., Dorey, N., Stumpp, M. et al. Long-term and trans-life-cycle effects of exposure to ocean acidification in the green sea urchin Strongylocentrotus droebachiensis . Mar Biol 160, 1835–1843 (2013). https://doi.org/10.1007/s00227-012-1921-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-012-1921-x