Abstract
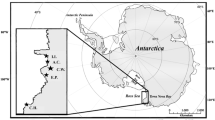
Leatherback turtles, Dermochelys coriacea, are highly migratory, spending most of their lives submerged or offshore where their feeding habits are difficult to observe. In order to elucidate the foraging ecology of leatherbacks off Massachusetts, USA, stable isotope analyses were performed on leatherback tissues and prey collected from 2005 to 2009. Stable isotope ratios of nitrogen and carbon were determined in whole blood, red blood cells, blood plasma, muscle, liver, and skin from adult male, female, and subadult leatherbacks. Isotopic values were analyzed by body size (curved carapace length) and grouped by sex, and groups were tested for dietary differences. Gelatinous zooplankton samples were collected from leatherback foraging grounds using surface dip nets and stratified net tows, and prey contribution to leatherback diet was estimated using a two-isotope Bayesian mixing model. Skin and whole blood δ13C values and red blood cell δ15N values were correlated with body size, while δ13C values of red blood cells, whole blood, and blood plasma differed by sex. Mixing model results suggest that leatherbacks foraging off Massachusetts primarily consume the scyphozoan jellyfishes, Cyanea capillata and Chrysaora quinquecirrha, and ctenophores, while a smaller proportion of their diet comes from holoplanktonic salps and sea butterflies (Cymbuliidae). Our results are consistent with historical observations of leatherback turtles feeding on scyphozoan prey in this region and offer new insight into size- and sex-related differences in leatherback diet.




Similar content being viewed by others
References
Abelson PH, Hoering TC (1961) Carbon isotope fractionation in formation of amino acids by photosynthetic organisms. Proc Natl Acad Sci USA 47:623–632
Arai MN (2005) Predation on pelagic coelenterates: a review. J Mar Biol Assoc UK 85:523–528
Belkin IM, Cornillon PC, Sherman K (2009) Fronts in large marine ecosystems. Prog Oceanogr 81:223–236
Benson SR, Forney KA, Harvey JT, Carretta JV, Dutton PH (2007) Abundance, distribution, and habitat of leatherback turtles (Dermochelys coriacea) off California, 1990–2003. Fish Bull 105:337–347
Bjorndal KA (1997) Foraging ecology and nutrition of sea turtles. In: Lutz PL, Musick JA (eds) The biology of sea turtles. CRC Press, Boca Raton, pp 199–231
Bleakney JS (1965) Reports of marine turtles from New England and Eastern Canada. Can Field Nat 79(2):120–128
Bostrom BL, Jones TT, Hastings M, Jones DR (2010) Behavior and physiology: the thermal strategy of leatherback turtles. PLoS ONE 5(11):e13925. doi:10.1371/journal.pone.0013925
Boulon RH, Dutton PH, McDonald DL (1996) Leatherback turtles (Dermochelys coriacea) on St. Croix, U.S. Virgin Islands: fifteen years of conservation. Chelon Conserv Biol 2:141–147
Brongersma LD (1969) Miscellaneous notes on turtles, IIA, IIB. Proc Koninklijke Nederlandse Akademie Wetenschappen (ser C) 72:76–102
Brudenall DK, Schwab IR, Fritsches KA (2008) Ocular morphology of the Leatherback sea turtle (Dermochelys coriacea). Vet Ophthalmol 11:99–110
Casey J, Garner J, Garner S, Williard A (2010) Diel foraging behavior of gravid leatherback sea turtles in deep water of the Caribbean Sea. J Exp Biol 213:3961–3971
Caut S, Guirlet E, Angulo E, Das K, Girondot M (2008) Isotope analysis reveals foraging area dichotomy for Atlantic leatherback turtles. PLoS Biol 3(3):e1845
Caut S, Angulo E, Courchamp F (2009) Variation in discrimination of factors (∆15N and (∆13C): the effect of diet isotopic values and applications for diet reconstruction. J Appl Ecol 46:443–453
Clarke A, Holmes LJ, Gore DJ (1992) Proximate and elemental composition of gelatinous zooplankton from the Southern Ocean. J Exp Mar Biol Ecol 155:55–68
Davenport J, Balazs GH (1991) Fiery bodies—are pyrosomas important items in the diet of leatherback turtles? Br Herp Soc B 37:33–38
Deibel D, Paffenhöfer GA (2009) Predictability of patches of neritic salps and doliolids (Tunicata, Thaliacea). J Plankton Res 31:1571–1579
den Hartog JC, Van Nierop MM (1984) A study on the gut contents of six leathery turtles Dermochelys coriacea (Linnaeus) (Reptilia: Testudines: Dermochelyidae) from British waters and from the Netherlands. Zool Verh 209:1–36
DeNiro MJ, Epstein S (1978) Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta 42:495–506
DeNiro MJ, Epstein S (1981) Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta 45:341–351
Desjardin NA (2005) Spatial, temporal, and dietary overlap of leatherback sea turtles (Dermochelys coriacea) and ocean sunfishes (family Molidae). Master Thesis, Florida Atlantic University, Boca Raton
Doyle TK, Houghton JDR, McDevitt R, Davenport J, Hays GC (2007) The energy density of jellyfish: estimates from bomb calorimetry and proximate-composition. J Exp Mar Biol Ecol 343:239–252
Duron M (1978) Contribution à létude de la biologie de Dermochelys coriacea (Linné) dans les Pertuis Charentais. Thése Universite Bordeaux 3me cycle 1465
Eckert SA (2002a) Distribution of juvenile leatherback sea turtle Dermochelys coriacea sightings. Mar Ecol Prog Ser 230:289–293
Eckert SA (2002b) Swim speed and movement patterns of gravid leatherback sea turtles (Dermochelys coriacea) at St. Croix, US Virgin Islands. J Exp Biol 205:3689–3697
Eckert SA, Eckert KL, Ponganis P, Kooyman GL (1989) Diving and foraging behavior by leatherback sea turtles. Can J Zool 67:2834–2840
Eisenberg JF, Frazier J (1983) A leatherback turtle (Dermochelys coriacea) feeding in the wild. J Herpetol 17(1):81–82
Ferraroli S, Georges JY, Gaspar P, Maho YL (2004) Endangered species: where leatherback turtles meet fisheries. Nature 429:521–522
France RL (1995) C-13 Enrichment in benthic compared to planktonic algae—foodweb implications. Mar Ecol Prog Ser 124:307–312
Fraser-Brunner A (1951) The ocean sunfishes (family Molidae). Bull Br Mus 1:89–121
Frazier J, Meneghel MD, Achaval F (1985) A clarification on the feeding habits of Dermochelys coriacea. J Herpetol 19(1):159–160
Fry B, Sherr EB (1984) δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems. Contrib Mar Sci 27:13–47
Gannes LZ, O’Brien DM, Martínez del Rio C (1997) Stable isotopes in animal ecology: assumptions, caveats, and a call for more laboratory experiments. Ecology 78:1271–1276
Gannes LZ, Martínez del Rio C, Koch P (1998) Natural abundance variations in stable isotopes and their potential uses in animal physiological ecology. Comp Biochem Physiol 119:725–737
Godley BJ, Thompson DR, Waldron S, Furness RW (1998) The trophic status of marine turtles as determined by stable isotope analysis. Mar Ecol Prog Ser 166:277–284
Graham WM, Pages F, Hammer WM (2001) A physical context for gelatinous zooplankton aggregations: a review. Hydrobiologia 451:199–212
Grant GS, Ferrell D (1993) Leatherback turtle, Dermochelys coriacea (Reptilia, Dermochelidae)—notes on near-shore feeding behavior and association with Cobia. Brimleyana 19:77–81
Hatase H, Takai N, Matsuzawa Y, Sakamoto W, Omuta K, Goto K, Arai N, Fujiwara T (2002) Size-related differences in feeding habitat use of adult female loggerhead turtles Caretta caretta around Japan determined by stable isotope analyses and satellite telemetry. Mar Ecol Prog Ser 233:273–281
Hatase H, Sato K, Yamaguchi M, Takahashi K, Tsukamoto K (2006) Individual variation in feeding habitat use by adult female green sea turtles (Chelonia mydas): are they obligately neritic herbivores? Oecologia 149:52–64
Hawkes LA, Broderick AC, Coyne MS, Godfrey MH, Lopez-Jurado LF, Lopez-Suarez P, Merino SE, Varo-Cruz N, Godley BJ (2006) Phenotypically linked dichotomy in sea turtle foraging requires multiple conservation approaches. Curr Biol 16:990–995
Hobson KA (1999) Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia 120:314–326
Hobson KA, Clark RG (1992) Assessing avian diets using stable isotopes II: factors influencing diet-tissue fractionation. Condor 94:189–197
Hobson KA, Alisauskas RT, Clark RG (1993) Stable-nitrogen isotope enrichment in avian tissues due to fasting and nutritional stress-implications for isotopic analyses of diet. Condor 95:388–394
Hobson KA, Piatt JF, Pitocchelli J (1994) Using stable isotopes to determine seabird trophic relationships. J Anim Ecol 63:786–798
Hooper SN, Paradis M, Ackman RG (1973) Distribution of trans-6-hexadecenoic acid, 7-methyl-7-hexadecenoic acid and common fatty acids in lipids of ocean sunfish Mola mola. Lipids 8:509–516
Houghton JDR, Doyle TK, Davenport J, Wilson RP, Hays GC (2008) The role of infrequent and extraordinary deep dives in leatherback turtles (Dermochelys coriacea). J Exp Biol 211:2566–2575
Innis C, Merigo C, Dodge K, Tlusty M, Dodge M, Sharp B, Myers A, McIntosh A, Wunn D, Perkins C, Herdt T, Norton T, Lutcavage M (2010) Health evaluation of leatherback turtles (Dermochelys coriacea) in the Northwestern Atlantic during direct capture and fisheries gear disentanglement. Chelonian Conserv Biol 9:205–222
James MC (2004) Dermochelys coriacea (leatherback sea turtle) penis display. Herpetol Rev 35(3):264
James MC, Herman TB (2001) Feeding of Dermochelys coriacea on medusae in the Northwest Atlantic. Chelonian Conserv Biol 4:202–205
James MC, Ottensmeyer CA, Myers RA (2005a) Identification of high-use habitat and threats to leatherback sea turtles in northern waters: new directions for conservation. Ecol Lett 8:195–201
James MC, Myers RA, Ottensmeyer CA (2005b) Behavior of leatherback sea turtles, Dermochelys coriacea, during the migratory cycle. Proc R Soc B 272:1547–1555
James MC, Sherrill-Mix SA, Myers RA (2007) Population characteristics and seasonal migrations of leatherback sea turtles at high latitudes. Mar Ecol Prog Ser 337:245–254
Johnson WS, Allen DM (2005) Zooplankton of the Atlantic and Gulf coasts : a guide to their identification and ecology. The John Hopkins University Press, Baltimore
Jones TT, Hastings MD, Bostrom BL, Pauly D, Jones DR (2011) Growth of captive leatherback turtles, Dermochelys coriacea, with inferences on growth in the wild: Implications for populations decine and recovery. J Exp Mar Biol Ecol 399:84–92
Kiljunen M, Grey J, Sinisalo T, Harrod C, Immonen H, Jones RI (2006) A revised model for lipid-normalizing δ13C values from aquatic organisms, with implications for isotope mixing models. J Appl Ecol 43:1213–1222
Larson RJ (1976) Marine flora and fauna of the northeastern United States. Cnidaria:Scyphozoa. NOAA Tech Rep NMFS Circ 397
Lazell JD (1980) New England waters: critical habitat for marine turtles. Copeia 1980:290–295
Logan J, Jardine T, Miller T, Bunn S, Cunjak R, Lutcavage M (2008) Lipid corrections in carbon and nitrogen stable isotope analyses: comparison of chemical extraction and modelling methods. J Anim Ecol 77:838–846
Longhurst AR (1998) Ecological geography of the sea. Academic Press, San Diego
Lutcavage ME (1996) Planning your next meal: leatherback travel routes and ocean fronts. In: Keinath JA, Barnard DE, Musick JA, Bell BA (eds) Proceedings of the 15th annual workshop on sea turtle biology and conservation. NOAA Tech Memo NMFS-SEFSC-387, p 355
MacGintie GE (1938) Notes on the natural history of some marine animals. Am Midl Nat 19:207–219
Madin LP, Kremer P, Wiebe PH, Purcell JE, Horgan EH, Nemazie DA (2006) Periodic swarms of the salp Salpa aspera in the slope waters of the NE United States: biovolume, vertical migration, grazing, and vertical flux. Deep Sea Res 53:804–819
Mann KH, Lazier JRN (2006) Dynamics of marine ecosystems: biological-physical interactions in the oceans, 3rd edn. Blackwell Science Inc., Cambridge
Martínez del Rio C, Wolf BO (2004) Mass-balance models for animal isotopic ecology. In: Starck JM, Wang T (eds) Physiological consequences of feeding. Springer, Berlin
McClellan CM, Braun-McNeill J, Avens L, Wallace BP, Read AJ (2010) Stable isotopes confirm a foraging dichotomy in juvenile loggerhead sea turtles. J Exp Mar Biol Ecol 387:44–51
Minagawa M, Wada E (1984) Stepwise enrichment of δ15N along food chains: further evidence of the relation between δ15N and animal age. Geochim Cosmochim Acta 48:1135–1140
Musick JA, Limpus CJ (1997) Habitat utilization and migration in juvenile sea turtles. In: Lutz PL, Musick JA (eds) The biology of sea turtles. CRC Press, Boca Raton, pp 137–163
Park R, Epstein S (1961) Metabolic fractionation of C13 & C12 in plants. Plant Physiol 36:133–138
Parnell AC, Inger R, Bearhop S, Jackson AL (2010) Source partitioning using stable isotopes: coping with too much variation. PLoS ONE 5(3):e9672. doi:10.1371/journal.pone.0009672
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Ann Rev Ecol Syst 18:293–320
Phillips DL, Gregg JW (2003) Source partitioning using stable isotopes: coping with too many sources. Oecologia 136:261–269
Phillips DL, Newsome SD, Gregg JW (2005) Combining sources in stable isotope mixing models: alternative methods. Oecologia 144:520–524
Pope EC, Hays GC, Thys TM, Doyle TK, Sims DW, Queiroz N, Hobson VJ, Kubicek L, Houghton JDR (2010) The biology and ecology of the ocean sunfish Mola mola: a review of current knowledge and future research perspectives. Rev Fish Biol Fish 20:471–487
Popp BN, Graham BS, Olson RJ, Hannides CCS, Lott MJ, López-Ibarra GA, Galván-Magaña F, Fry B (2007) Insight into the trophic ecology of yellowfin tuna, Thunnus albacares, from compound-specific nitrogen isotope analysis of proteinaceous amino acids. In: Dawson T, Seigwolf R (eds) Isotopes as tracers of ecological change. Elsevier, San Diego, pp 173–190
Post DM, Layman CA, Arrington DA, Takimoto G, Quattrochi J, Montaña CG (2007) Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152:179–189
Pritchard PCH (1973) International migrations of South American sea turtles (Cheloniidae and Dermochelyidae). Anim Behav 21:18–27
R Development Core Team (2008) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rau GH, Sweeney RE, Kaplan IR (1982) Plankton 13C: 12C ratio changes with latitude: differences between northern and southern oceans. Deep Sea Res 29:1035–1039
Reich KJ, Bjorndal KA, Bolten AB (2007) The ‘lost years’ of green turtles: using stable isotopes to study cryptic lifestages. Biol Lett 3:712–714
Reich KJ, Bjorndal KA, Martínez del Rio C (2008) Effects of growth and tissue type on the kinetics of 13C and 15 N incorporation in a rapidly growing ectotherm. Oecologia 155:651–663
Reich KJ, Bjorndal KA, Frick MG, Witherington BE, Johnson C, Bolten AB (2009) Polymodal foraging in adult female loggerheads (Caretta caretta). Mar Biol 157:113–121
Rooker JR, Turner JP, Holt SA (2006) Trophic ecology of Sargassum-associated fishes in the Gulf of Mexico determined from stable isotopes and fatty acids. Mar Ecol Prog Ser 313:249–259
Saino T, Hattori A (1987) Geographic variation of the water column distribution of suspended particulate nitrogen and its 15N natural abundance in the Pacific and its marginal seas. Deep Sea Res 34:807–827
Sale A, Luschi P, Mencacci R, Lambardi P, Hughes GR, Hays GC, Benvenuti S, Papi F (2006) Long-term monitoring of leatherback turtle dive behaviour during oceanic movements. J Exp Mar Biol Ecol 328:197–210
Seminoff JA, Bjorndal KA, Bolten AB (2007) Stable carbon and nitrogen isotope discrimination and turnover in Pond Sliders Trachemys scripta: insights for trophic study of freshwater turtles. Copeia 2007:534−542
Seminoff JA, Jones TT, Eguchi T, Hastings M, Jones DR (2009) Stable carbon and nitrogen isotope discrimination in soft tissues of the leatherback turtle (Dermochelys coriacea): insights for trophic studies of marine turtles. J Exp Mar Biol Ecol 381:33–41
Shillinger GL, Palacios DM, Bailey H, Bograd SJ, Swithenbank AM, Gaspar P, Wallace BP, Spotila JR, Paladino FV, Peidra R, Eckert SA, Block BA (2008) Persistent leatherback turtle migrations present opportunities for conservation. PLoS Biol 6(7):e171
Sweeting CJ, Barry J, Barnes C, Polunin NVC, Jennings S (2007a) Effects of body size and environment on diet-tissue δ 15N fractionation in fishes. J Exp Mar Biol Ecol 340:1–10
Sweeting CJ, Barry J, Polunin NVC, Jennings S (2007b) Effects of body size and environment on diet-tissue δ 13C fractionation in fishes. J Exp Mar Biol Ecol 352:165–176
TEWG (Turtle Expert Working Group) (2007) An assessment of the leatherback turtle population in the Atlantic Ocean. NOAA Tech Memo NMFS-SEFSC-555
Tieszen LL, Boutton TW, Tesdahl KG, Slade NA (1983) Fractionation and turnover of stable carbon isotopes in animal tissues: implications for δ13C analysis of diet. Oecologia 57:32–37
Vanderklift MA, Ponsard S (2003) Sources of variation in consumer-diet δ15N enrichment: a meta-analysis. Oecologia 136:169–182
Wada E, Hattori A (1991) Nitrogen in the sea: forms, abundances, and rate processes. CRC Press, Boca Raton
Wallace BP, Seminoff JA, Kilham SS, Spotila JR, Dutton PH (2006a) Leatherback turtles as oceanographic indicators: stable isotope analyses reveal a trophic dichotomy between ocean basins. Mar Biol 149:953–960
Wallace BP, Kilham SS, Paladino FV, Spotila JR (2006b) Energy budget calculations indicate resource limitation in eastern Pacific leatherback turtles. Mar Ecol Prog Ser 318:263–270
Wallace BP, Avens L, Braun-McNeill J, McClellan CM (2009) The diet composition of immature loggerheads: insights on trophic niche, growth rates, and fisheries interactions. J Exp Mar Biol Ecol 373:50–57
Wiebe PH, Burt KH, Boyd SH, Morton AW (1976) A multiple opening/closing net and environmental sensing system for sampling zooplankton. J Mar Res 34:313–326
Wiebe PH, Morton AW, Bradley AM, Backus RH, Craddock JE, Barber V, Cowles TJ, Flierl GR (1985) New developments in the MOCNESS, an apparatus for sampling zooplankton and micronekton. Mar Biol 87:313–323
Witt MJ, Broderick AC, Johns DJ, Martin C, Penrose R, Hoogmoed MS, Godley BJ (2007) Prey landscapes help identify potential foraging habitats for leatherback turtles in the NE Atlantic. Mar Ecol Prog Ser 337:231–244
Wolf N, Carleton SA, Martínez del Rio C (2009) Ten years of experimental animal isotopic ecology. Funct Ecol 23:17–26
Acknowledgments
We are indebted to many people for making this research possible: C. Merigo, C. Innis, A. Myers, M. Dodge, G. Purmont, M. Leach, B. Sharp, S. Landry, M. Murphy, G. Tomasian, N. Fragoso, K. Sampson, R. Smolowitz, K. Hirokawa, J. Casey, S. Leach, J. Wilson, E. Eldredge, M. Dodd, T. Norton, M. Zani, T. Naessig, K. Sutherland, K. Houtler, I. Hanley, and the late J. Vickery and his crew of the R/V Marguerite. Special thanks to P. Wiebe for lending us his MOCNESS and for his expert advice on running it. We thank A. Ouimette and the staff of the UNH Stable Isotope Lab for assistance with isotope analyses. B. Stacy and J. Wyneken kindly provided samples from stranded Florida leatherbacks. The authors acknowledge use of SEATURTLE.ORG’s Maptool program (http://www.seaturtle.org/maptool/). This work was conducted under the authority of the National Marine Fisheries Service Endangered Species Act Section 10 Permit #1557-03 and University of New Hampshire IACUC #060501, and funded by National Oceanic and Atmospheric Administration Grant #NA04NMF4550391 and National Fish and Wildlife Foundation Grant #2008-0076-000 to M. Lutcavage. Turtle disentanglement was conducted under the authority of NOAA 50 CFR Part 222.310 and supported by the Massachusetts Division of Marine Fisheries through the National Oceanic and Atmospheric Administration Grant #NA07NMF4720052. Additional funding was provided by the Cape Cod Commercial Hook Fishermen’s Association. K. D. was supported by a UNH Marine Program Fellowship. We thank H. Haas, J. Houghton, B. Wallace, and one anonymous reviewer for comments that improved earlier drafts of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. D. R. Houghton.
Rights and permissions
About this article
Cite this article
Dodge, K.L., Logan, J.M. & Lutcavage, M.E. Foraging ecology of leatherback sea turtles in the Western North Atlantic determined through multi-tissue stable isotope analyses. Mar Biol 158, 2813–2824 (2011). https://doi.org/10.1007/s00227-011-1780-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-011-1780-x