Abstract
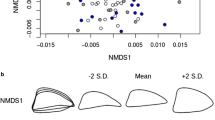
The extent to which genetic divergence can occur in the absence of physical barriers to gene flow is currently one of the most controversial topics in evolutionary biology, with implications for our understanding of speciation, phenotypic plasticity and adaptive potential. This is illustrated by a recent study reporting a surprising pattern of genetic differentiation between intertidal and subtidal morphotypes of the broadcast-spawning Antarctic limpet Nacella concinna. To explore this further, we collected almost 400 Antarctic limpets from four depths (intertidal, 6, 15 and 25 m) at Adelaide island, Antarctica, and conducted a combined morphometric and genetic analysis using 168 polymorphic amplified fragment length polymorphism (AFLP) loci. Morphological analysis revealed not only pronounced differences between the two morphotypes, but also a continuous cline in shell shape from the intertidal zone down to 25 m depth, suggesting that the distinction between the morphotypes may be artificial. Moreover, genetic analysis using both F st and a Bayesian analogue found no evidence for differentiation either between the two morphotypes or by depth, and a Bayesian cluster analysis did not detect any cryptic genetic structure. Our findings lend support to the notion that limpets can be phenotypically highly plastic, although further studies are required to determine unequivocally whether there is any genetic basis to the observed variation in shell morphology.



Similar content being viewed by others
References
Ajmone-Marsan J, Valentini A, Cassandro M, Vecchiotti-Antaldi G, Bertoni G, Kuiper M (1997) AFLPTM markers for DNA fingerprinting in cattle. Anim Genet 28:418–426
Baxter JM (1983) Allometric relationships of Patella vulgata L. Shell characters at three adjacent sites at Sandwick Bay in Orkney. J Nat Hist 17:743–755
Beaumont AR, Wei JHC (1991) Morphological and genetic variation in the Antarctic Limpet Nacella concinna (Strebel, 1908). J Molluscan Stud 57:443–450
Bensch S, Akesson M (2005) Ten years of AFLP in ecology and evolution: why so few animals? Mol Ecol 14:2899–2914
Bonin A, Bellemain E, Bronken Eidesen P, Pompanon F, Brochmann C, Taberlet P (2004) How to track and assess genotyping errors in population genetic studies. Mol Ecol 13:3261–3273
Bonin A, Ehrich D, Manel S (2007) Statistical analysis of amplified fragment length polymorphism data: a toolbox for molecular ecologists and evolutionists. Mol Ecol 16:3737–3758
Bowden DA, Clarke A, Peck LS, Barnes DKA (2006) Antarctic sessile marine benthos: colonisation and growth on artificial substrata over 3 years. Mar Ecol Prog Ser 316:1–16
Branch GM, Marsh AC (1978) Tenacity and shell shape in six Patella species: adaptive features. J Exp Mar Biol Ecol 34:111–130
Brown KM, Fraser KPP, Barnes DKA, Peck LS (2004) Ice scour frequency dictates Antarctic shallow-water community structure. Oecologia 141:121–129
Butlin RK, Galindo J, Grahame JW (2008) Sympatric, parapatric or allopatric: the most important way to clasify speciation? Philos Trans R Soc Lond B Biol Sci 363:2997–3007
Caballero A, Quesada H, Rolán-Alvarez E (2008) Impact of AFLP fragment size homoplasy on the estimation of population genetic diversity and the detection of selective loci. Genetics 179:539–554
Cavers S, Degen B, Caron H, Lemes MR, Margis R, Salgueiro F, Lowe AJ (2005) Optimal sampling strategy for estimation of spatial genetic structure in tree populations. Heredity 95:281–289
Crampton JS, Haines AJ (1996) Users’ manual for programs HANGLE, HMATCH and HCURVE for the Fourier shape analysis of two-dimensional outlines. Institute of Geological and Nuclear Sciences Science Report 96. 37:1–28
Crampton JS, Maxwell PA (2000) Size: all it’s shaped up to be? Evolution of shape through the lifespan of the Cenozoic bivalve Spissatella (Crassatellidae). In: Harper EM, Taylor JD, Crame JA (eds) The evolutionary biology of the Bivalvia. The Geological Society of London, London, pp 399–423
Crawley MJ (2002) Statistical computing, an introduction to data analysis using S-plus. Wiley, Chichester
Dasmahapatra KK, Hoffman JI, Amos W (2009) Pinniped phylogenetic relationships inferred using AFLP markers. Heredity 103:168–177
de Aranzamendi MC, Sahade R, Tatian M, Chiappero MB (2008) Genetic differentiation between morphotypes in the Antarctic limpet Nacella concinna as revealed by inter-simple sequence repeat markers. Mar Biol 154:875–885
de Wolf H, Backeljau T, Madeiros R, Verhagen R (1997) Microgeographical shell variation in Littorina striata, a planktonic developing periwinkle. Mar Biol 129:331–342
de Wolf H, Backeljau T, Verhagen R (1998a) Congruence between allozyme and RAPD data in assessing macrogeographical genetic variation in the periwinkle Littorina striata (Mollusca, Gastropoda). Heredity 81:486–492
de Wolf H, Backeljau T, Verhagen R (1998b) Spatio-temporal genetic structure and gene flow between two distinct shell morphs of the planktonic developing periwinkle Littorina striata (Mollusca: Prosobranchia). Mar Ecol Prog Ser 163:155–163
Dyer AT, Leonard KJ (2000) Contamination, error, and nonspecific molecular tools. Phytopathology 90:565–567
Falush D, Stephens M, Pritchard JK (2003) Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164:1567–1587
Falush D, Stephens M, Pritchard JK (2007) Inference of population structure using multilocus genotype data: dominant markers and null alleles. Mol Ecol Notes 7:574–578
Galindo J, Morán P, Rolán-Alvarez E (2009) Comparing geographical genetic differentiation between candidate and non candidate loci for adaptation strengthens support for parallel ecological divergence in the marine snail Littorina saxatilis. Mol Ecol 18:919–930
Haines AJ, Crampton JS (2000) Improvements to the method of Fourier shape analysis as applied in morphometric studies. Palaeontology 43:765–783
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4:4–9
Harley CDG, Denny MW, Mach KJ, Miller LP (2008) Thermal stress and morphological adaptations in limpets. Funct Ecol. doi:10.1111/j.1365-2435.2008.01496.x
Hockey PAR, Branch GM (1983) Do oystercatchers influence limpet shell shape? Veliger 26:139–141
Hoffman JI, Amos W (2005) Microsatellite genotyping errors: detection approaches, common sources and consequences for paternal exclusion. Mol Ecol 14:599–612
Hollander J, Lindergarth M, Johannesson K (2005) Local adaptation but not geographical separation promotes assortative mating in a snail. Anim Behav 70:1209–1219
Holsinger KE, Wallace LE (2004) Bayesian approaches for the analysis of population genetic structure: an example from Platanthera leucophaea (Orchidaceae). Mol Ecol 13:887–894
Holsinger KE, Lewis PO, Dey DK (2002) A bayesian approach to inferring population structure from dominant markers. Mol Ecol 11:1157–1164
Janson K (1982) Genetic and environmental effects on the growth rate of Littorina saxatilis. Mar Biol 69:73–78
Johannesson K (2003) Evolution in Littorina: ecology matters. J Sea Res 49:107–117
Johannesson B, Johannesson K (1996) Population differences in behaviour and morphology in the snail Littorina saxatilis: phenotypic plasticity or genetic differentiation? J Zool 240:475–493
Kaiser HF (1960) The application of electronic computers to factor analysis. Educ Psychol Measur 20:141–151
Kemp P, Bertness MD (1984) Snail shape and growth rates: evidence for plastic shell allometry in Littorina littorea. Proc Natl Acad Sci USA 81:811–813
Krauss SL (2000) Accurate gene diversity estimates from amplified fragment length polymorphism (AFLP) markers. Mol Ecol 9:1241–1245
Latch EK, Dharmarajan G, Glaubitz JC, Rhodes OE Jr (2006) Relative performance of Bayesian clustering software for inferring population substructure and individual assignment at low levels of population differentiation. Conserv Genet 7:295–302
Lynch M, Milligan BG (1994) Analysis of population genetic structure with RAPD markers. Mol Ecol 3:91–99
Maughan PJ, Saghai Maroof MA, Buss GR, Huestis GM (1996) Amplified fragment length polymorphism (AFLP) in soybean: species diversity, inheritance, and near-isogenic line analysis. Theor Appl Genet 93:392–401
Menge BA, Branch GM (2001) Rocky intertidal communities. In: Bertness MD, Gaines SD, Hay ME (eds) Marine Community Ecology. Sinauer Associates, Sunderland, pp 221–251
Meudt HM, Clarke AC (2007) Almost forgotten or latest practice? AFLP applications, analyses and advances. Trends Plant Sci 12:106–108
Miller SL (1974) Adaptive design of locomotion and foot form in prosobranch gastropods. J Exp Mar Biol Ecol 14:99–156
Moore HB (1934) The relation of shell growth to environment in Patella vulgata. Proc Malacological Soc London 21:217–222
Mueller UG, Wolfenbarger LL (1999) AFLP genotyping and fingerprinting. Trends Ecol Evol 14:389–394
Nolan CP (1991) Size, shape and shell morphology in the Antarctic Limpet Nacella concinna at Signy island, South Orkney islands. J Molluscan Stud 57:225–238
Orton JH (1928) Observations on Patella vulgata. II. Rate of growth of shell. J Mar Biol Ass UK 15:663–674
Palumbi SR (1999) All males are not created equal: fertility differences depend on gamete recognition polymorphisms in sea urchins. Proc Natl Acad Sci USA 96:12632–12637
Panova M, Hollander J, Johannesson K (2006) Site-specific divergencein parallel hybrid zones suggests nonallopatric evolution of reproductive barriers. Mol Ecol 15:4021–4031
Parsons KE (1997) Role of dispersal ability in the phenotypic differentiation and plasticity of two marine gastropods. Oecologia 110:461–471
Peck LS, Convey P, Barnes DKA (2006) Environmental constraints on life histories in Antarctic ecosystems: tempos, timings and predictability. Biol Rev 81:75–109
Picken GB (1980) Distribution, growth, and reproduction of the Antarctic Limpet Nacella (Patinigera) concinna (Strebel, 1908). J Exp Mar Biol Ecol 42:71–85
Pickles AR, Grahame J (1999) Mate choice in divergent morphs of the gastropod mollusc Littorina saxatilis (Olivi): speciation in action? Anim Behav 58:181–184
Polisky B, Greene P, Garfin DE, McCarthy BJ, Goodman HM, Boyer HW (1975) Specificity of substrate recognition by the EcoRI restriction endonuclease. Proc Natl Acad Sci USA 72:3310–3314
Powell AWB (1951) Antarctic and subanctarctic Mollusca: pelecypoda and gastropoda. Discov Rep (USA) 26:49–196
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
R Development Team (2005) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rasband W (2008) http://rsbweb.nih.gov/ij, National Institute of Health, USA
Rolán-Alvarez E, Erlandsson J, Johannesson K, Cruz R (1999) Mechanisms of incomplete prezygotic isolation in an intertidal snail; testing behavioural models in wild populations. J Evol Biol 12:879–890
Rolán-Alvarez E, Carballo M, Galindo J, Morán P, Fernandez B, Caballero A, Cruz R, Boulding EG, Johannesson K (2004) Nonallopatric and parallel origin of local reproductive barriers between two snail morphotypes. Mol Ecol 13:3415–3424
Seeley RH (1986) Intense natural selection caused a rapid morphological transition in a living marine snail. Proc Natl Acad Sci USA 83:6897–6901
Shabica SV (1976) The natural history of the Antarctic limpet Patinigera polaris (Humbron & Jacquinot)
Stanwell-Smith D, Clarke A (1998) The timing of reproduction in the Antarctic limpet Nacella concinna (Strebel, 1908) (Patellidae) at Signy island, in relation to environmental variables. J Molluscan Stud 64:123–127
Strebel H (1908) Dei Gastropoden. Wissenschaftliche Ergebnisse der Schwedischen Südpolar-Expedition, 1901–1903. 6:1–112
Swanson WJ, Aquadro CF, Vacquier VD (2001) Polymorphism in abalone fertilization proteins is consistent with the neutral evolution of the egg’s receptor for lysin (VERL) and positive Darwinian selection of sperm lysin. Mol Biol Evol 18:376–383
Trussell GC, Smith LD (2000) Induced defenses in response to an invading crab predator: an explanation of historical and geographic phenotypic change. Proc Natl Acad Sci USA 97:2123–2127
Vekemans X (2002) AFLP-SURV version 1.0. Distributed by the author. Laboratoire de Génétique et Ecologie Végétale. Université Libre de Bruxelles, Belgium
Vermeij GJ (1973) Morphological patterns in high-intertidal gastropods—adaptive strategies and their limitations. Mar Biol 20:319–346
Vos P, Hogers R, Bleker M, Reijans M, Van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Wagner GP, Kenney-Hunt JP, Pavlicev M, Peck JR, Waxman D, Cheverud JM (2008) Pleiotropic scaling of gene effects and the ‘cost of complexity’. Nature 452:470–473
Walker AJM (1972) Introduction to the ecology of the Antarctic limpet Patinigera polaris (Hombron and Jaquinot) at Signy island, South Orkney islands. Br Antarctic Surv Bull 28:49–71
Waller CL, Worland MR, Convey P, Barnes DKA (2006) Ecophysiological strategies of Antarctic intertidal invertebrates faced with freezing stress. Polar Biol 29:1077–1083
Warburton K (1976) Shell form, behaviour, and tolerance to water movement in the limpet Patina pellucida (L.)(Gastropoda: Prosobranchia). J Exp Marine Biol Ecol 23:307–325
Weihe E, Abele D (2008) Differences in the physiological response of inter- and subtidal Antarctic limpets Nacella concinna to aerial exposure. Aquatic Biol 4:155–166
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Wilding CS, Butlin RK, Grahame J (2001) Differential gene exchange between parapatric morphs of Littorina saxatilis detected using AFLP markers. J Exp Biol 14:611–619
Zhivotovsky LA (1999) Estimating population structure in diploids with multilocus dominant DNA markers. Mol Ecol 8:907–913
Acknowledgments
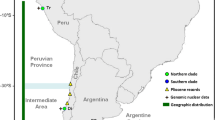
This paper was produced within the BAS Q4 BIOREACH/BIOFLAME core program. The authors would like to thank the Rothera Dive Team for providing samples, Peter Fretwell for making Figure 1 and Pete Rothery for statistical advice and Kanchon Dasmahapatra, Geerat Vermeij and three anonymous referees for helpful comments that improved the manuscript. Overall diving support was provided by the NERC National Facility for Scientific Diving at Oban. JH was supported by a Natural Environment Research Council (NERC) British Antarctic Survey (BAS) Strategic Alliance Fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Reusch.
Rights and permissions
About this article
Cite this article
Hoffman, J.I., Peck, L.S., Hillyard, G. et al. No evidence for genetic differentiation between Antarctic limpet Nacella concinna morphotypes. Mar Biol 157, 765–778 (2010). https://doi.org/10.1007/s00227-009-1360-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-009-1360-5