Abstract
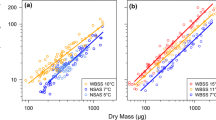
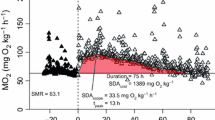
Rates of routine respiration (RR, μl O2 fish−1 h−1) and total ammonia nitrogen excretion (ER, μg NH4–N + NH3–N fish−1 h−1) were measured on larval and juvenile haddock (Melanogrammus aeglefinus) to ascertain how energy losses due to metabolism were influenced by temperature (T), dry body mass (MD, mg) and specific growth rate (SGR, % per day). RR and ER increased with MD according to y = a · MDb with b-values of 0.96, 0.98, 1.14, and 0.89, 0.78, 0.74, respectively, at 10, 7, and 4°C, respectively. Multiple regressions explained 98% of the variability in the combined effects of MD and T on RR and ER in larval haddock: RR = 0.97 · MD0.98 · e0.092 · T; ER = 0.06 · MD0.79 · e0.092 · T. In young juvenile (24–30 mm standard length) haddock, RR tended to decline (P = 0.06) and ER significantly declined (P = 0.02) with increasing SGR. O:N ratios significantly increased with increasing SGR suggesting that N was spared in relatively fast-growing individuals. Our results for young larval and juvenile haddock suggest: (1) nearly isometric scaling of RR with increasing body size, (2) allometric scaling of ER with increasing body size, (3) Q10 values of 2.5 for both RR and ER, (4) metabolic differences in substrate utilization between relatively fast- and slow-growing individuals, and (5) that rates of routine energy loss and growth were not positively related. The measurements in this study will provide robust parameter estimates for individual-based models that are currently being utilized to investigate how variability in climatic forcing influences the vital rates of early life stages of haddock. Our results also stress that inter-individual differences in rates of energy loss should not be overlooked as a factor influencing growth variability among individuals.





Similar content being viewed by others
References
Bailey KM, Houde ED (1989) Predation on eggs and larvae of marine fishes and the recruitment problem. Adv Mar Biol 25:1–83. doi:10.1016/S0065-2881(08)60187-X
Blaikie HB, Kerr SR (1996) Effect of activity level on apparent heat increment in Atlantic cod, Gadus morhua. Can J Fish Aquat Sci 53:2093–2099. doi:10.1139/cjfas-53-9-2093
Bochdansky AB, Leggett WC (2001) Winberg revisited: convergence of routine metabolism in larval and juvenile fish. Can J Fish Aquat Sci 58:220–230. doi:10.1139/cjfas-58-1-220
Bower CE, Holm-Hansen T (1980) A salicylate-hypochlorite method for determining ammonia in seawater. Can J Fish Aquat Sci 37:794–798
Brett JR, Groves TDD (1979) Physiological energetics. In: Hoar WS, Randall DJ, Brett JR (eds) Fish physiology, vol VIII, bioenergetics and growth. Academic Press, New York, pp 279–351
Brown JH, Gillooly JF, Allen AP, Savage VM, West GB (2004) Toward a metabolic theory of ecology. Ecology 85:1771–1789. doi:10.1890/03-9000
Buckley LJ, Lough RG, Peck MA, Werner FE (2000) Larval Atlantic cod and haddock growth models, metabolism, ingestion, and temperature effects. Can J Fish Aquat Sci 57(9):1957–1960. doi:10.1139/cjfas-57-9-1957
Buckley LJ, Caldarone EM, Lough RG (2004) Optimum temperature and food-limited growth of larval Atlantic cod (Gadus morhua) and haddock (Melanogrammus aeglefinus) on Georges Bank. Fish Oceanogr 13:134–140. doi:10.1046/j.1365-2419.2003.00278.x
Chambers RC, Waiwood KG (1996) Maternal and seasonal differences in egg sizes and spawning characteristics of captive Atlantic cod Gadus morhua. Can J Fish Aquat Sci 53:1986–2003. doi:10.1139/cjfas-53-9-1986
Clarke A, Johnston NM (1999) Scaling of metabolic rate with body mass and temperature in teleost fish. J Anim Ecol 68:893–905. doi:10.1046/j.1365-2656.1999.00337.x
Clemmesen C, Bühler V, Carvalho G, Case R, Evans G, Hauser L et al (2003) Variability in condition and growth of Atlantic cod larvae and juveniles reared in mesocosms: environmental and maternal effects. J Fish Biol 62(3):706–723. doi:10.1046/j.1095-8649.2003.00060.x
Cody RP, Smith JK (2006) Applied statistics and the SAS programming language. Pearson Prentice Hall, Upper Saddle River
Conover DO, Present TMC (1990) Countergradient variation in growth rate: compensation for length of the growing season among Atlantic silversides from different latitudes. Oecologia 83:316–324
Czekajewski J, Nennerfelt L, Kaczmarek H, Rabek JF (1994) Application of a new generation of computerized apparatus for the study of oxygen uptake and production of CO and CO2 during photo-(thermal) oxidation of polymers. Acta Polym 45:369–374. doi:10.1002/actp.1994.010450505
Cutts CJ, Metcalfe NB, Taylor AC (1998) Aggression and growth depression in juvenile Atlantic salmon: The consequences of individual variation in standard metabolic rate. J Fish Biol 52:1026–1037. doi:10.1111/j.1095-8649.1998.tb00601.x
Fahay MP, Berrien PL, Johnson DL, Morse WW (1999) Essential fish habitat source document: Atlantic cod Gadus morhua life history and habitat characteristics. NOAA Tech Mem NMFS NE, no. 124 (not paginated)
Finn RN, Rønnestad I, van der Meeren T, Fyhn HJ (2002) Fuel and metabolic scaling during the early life stages of Atlantic cod Gadus morhua. Mar Ecol Prog Ser 243:217–234. doi:10.3354/meps243217
Fry FEJ (1957) The aquatic respiration of fish. In: Brown ME (ed) The physiology of fishes, vol 1, metabolism. Academic Press, New York
Green J, Jones R, Brownell S (2004) Age and growth of larval cod and haddock on Georges Bank during 1995 and 1996. Mar Ecol Prog Ser 283:255–268. doi:10.3354/meps283255
Gregory TR, Wood CM (1998) Individual variation and interrelationships between swimming performance, growth rate, and feeding in juvenile rainbow trout (Oncorhynchus mykiss). Can J Fish Aquat Sci 55:1583–1590. doi:10.1139/cjfas-55-7-1583
Giguère LA, Côté B, St-Pierre J-F (1988) Metabolic rates scale isometrically in larval fishes. Mar Ecol Prog Ser 50:13–19. doi:10.3354/meps050013
Houde ED (1999) Mortality. In: Fuiman LA, Werner RR (eds) Fishery science: the unigue contributions of early life stages. Blackwell, Oxford, pp 64–87
Jobling M (1981) The influences of feeding on the metabolic rate of fishes: a short review. J Fish Biol 18:385–400. doi:10.1111/j.1095-8649.1981.tb03780.x
Kiørboe T, Munk P, Richardson K (1987) Respiration and growth of larval herring Clupea harengus: relation between specific dynamic action and growth efficiency. Mar Ecol Prog Ser 40:1–10. doi:10.3354/meps040001
Klumb R, Rudstram LG, Mills EL (2003) Comparison of alewife young-of-the-year and adult respiration and swimming speed bioenergetics model parameters: Implications of extrapolation. Trans Am Fish Soc 132:1089–1103. doi:10.1577/T03-038
Kolok AS, Oris JT (1995) The relationship between specific growth rate and swimming performance in male fathead minnows (Pimephales promelas). Can J Zool 73:2165–2167. doi:10.1139/z95-254
Laurence GC (1978) Comparative growth, respiration and delayed feeding abilities of larval cod (Gadus morhua) and haddock (Melanogrammus aeglefinus) as influenced by temperature during laboratory studies. Mar Biol (Berl) 50:1–7. doi:10.1007/BF00390536
Leising AW, Franks PJS (1999) Larval Atlantic cod (Gadus morhua) and haddock (Melanogrammus aeglefinus) growth on Georges Bank: a model with temperature, prey size, and turbulence forcing. Can J Fish Aquat Sci 56:25–36. doi:10.1139/cjfas-56-1-25
Lough RG, Caldarone EM, Rotunno TK, Broughton EA, Burns BR, Buckley LJ (1996) Vertical distribution of cod and haddock eggs and larvae, feeding and condition in stratified and mixed waters on southern Georges Bank, May 1992. Deep Sea Res Part II Top Stud Oceanogr 43:1875–1904. doi:10.1016/S0967-0645(96)00053-7
Martell DJ, Kieffer JD, Trippel EA (2005) Effects of temperature during early life history on embryonic and larval development and growth in haddock. J Fish Biol 66:1558–1575. doi:10.1111/j.0022-1112.2005.00699.x
Marteinsdottir G, Steinarsson A (1998) Maternal influence on the size and viability of Iceland cod Gadus morhua eggs and larvae. J Fish Biol 52(6):1241–1258
Mayzaud R, Conover J (1988) O:N atomic ratio as a tool to describe zooplankton metabolism. Mar Ecol Prog Ser 45:289–302. doi:10.3354/meps045289
Otterlei E, Nyhammer G, Folkvord A, Stefansson SO (1999) Temperature- and size-dependent growth of larval and juvenile Atlantic cod (Gadus morhua): a comparative study of Norwegian coastal cod and north-east Atlantic cod. Can J Fish Aquat Sci 56:2099–2111. doi:10.1139/cjfas-56-11-2099
Ottersen G, Loeng H (2000) Covariability in early growth and year-class strength of Barents Sea cod, haddock, and herring: the environmental link. ICES J Mar Sci 57:339–348. doi:10.1006/jmsc.1999.0529
Peck MA, Buckley LJ (2008) Measurements of larval Atlantic cod (Gadus morhua) routine metabolism and individual-based modeling. J Appl Ichthyology 24:144–149. doi:10.1111/j.1439-0426.2007.01004.x
Peck MA, Daewel U (2007) Physiologically-based limits to larval fish food consumption and growth: Implications for individual-based models. Mar Ecol Prog Ser 347:171–183. doi:10.3354/meps06976
Peck MA, Buckley LJ, Bengtson DA (2004) Inter-individual differences in rates of routine energy loss and growth in young-of-year juvenile Atlantic cod (Gadus morhua). J Fish Biol 64:984–995. doi:10.1111/j.1095-8649.2004.00366.x
Peck MA, Buckley LJ, Calderone EM, Bengtson DA (2003) Effects of food consumption and temperature on growth rate and biochemical-based indicators of growth in early juvenile Atlantic cod Gadus morhua and haddock Melanogrammus aeglefinus. Mar Ecol Prog Ser 251:233–243. doi:10.3354/meps251233
Peck MA, Buckley LJ, Bengtson DA (2005) Effects of temperature, body size, and feeding on rates of metabolism in juvenile haddock, Melanogrammus aeglefinus. J Fish Biol 66:911–923. doi:10.1111/j.0022-1112.2005.00633.x
Post JR, Lee JA (1996) Metabolic ontogeny of teleost fishes. Can J Fish Aquat Sci 53:910–923. doi:10.1139/cjfas-53-4-910
Sogard SM (1997) Size-selective mortality in the juvenile stage of teleost fishes: A review. Bull Mar Sci 60:1129–1157
Theilacker GH, Kimball AS (1984) Comparative quality of rotifers and copepods as food for larval fishes. Calif Coop Ocean Fish Invest Rep 15:80–86
Werner F, Perry RI, Lough RG, Naimie CE (1996) Trophodynamic and advective influences on Georges Bank larval cod and haddock. Deep Sea Res Part II Top Stud Oceanogr 43:1793–1822. doi:10.1016/S0967-0645(96)00042-2
Acknowledgments
We would like to thank the employees of the US NOAA NMFS Narragansett Marine Laboratory for the use of equipment and rearing facilities. We are particularly grateful to E. Davies and P. Samson for maintaining the haddock broodstock and rearing the haddock early life stages. We would also like to thank M.-L. Dickson for her help with the MicroOxymax dual gas respirometer, and E. Caldarone and J. Specker for their help with laboratory analyses. Valuable comments were received from three anonymous reviewers. The research was funded by US NOAA NMFS Cooperative Marine Education and Research Program (#NA04NMF4550377) awarded to DAB and MAP. Partial funding for this research was received from the “GLOBEC-Germany” program (FKZ 03FO320E).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H.O. Pörtner.
Rights and permissions
About this article
Cite this article
Lankin, K.F., Peck, M.A., Buckley, L.J. et al. The effects of temperature, body size and growth rate on energy losses due to metabolism in early life stages of haddock (Melanogrammus aeglefinus). Mar Biol 155, 461–472 (2008). https://doi.org/10.1007/s00227-008-1043-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-008-1043-7