Abstract
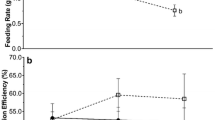
The rocky subtidal community off the Atlantic coast of Nova Scotia has historically undergone a cyclical transition between Laminaria-dominated kelp beds and sea urchin-dominated barrens. Since the introduction of the invasive alga Codium fragile ssp. tomentosoides, a third community state has emerged: Codium-dominated algal beds. We conducted a 42-week feeding experiment in the laboratory, which mimicked the quantity and quality of food available to urchins (Strongylocentrotus droebachiensis) in each of these community states. Feeding rate, growth, reproduction, and survival of urchins fed either Laminaria longicruris or C. fragile ad libidum, or L. longicruris 2 days per month, were measured. Although the ad libidum feeding rate on C. fragile was higher than that on kelp, energy intake was lower. Urchins in the ad libidum kelp treatment were larger and had larger gonads than those in the C. fragile treatment. Urchins fed kelp infrequently exhibited little somatic and gonadic growth over the course of the experiment. Regression analysis revealed that urchin performance on these diets was strongly related to energy intake. Diet treatment had no effect on survival or gonad maturation. Although urchins can consume substantial amounts of C. fragile, it appears that they cannot, or do not, feed quickly enough to compensate for its lower nutritional value. Our results suggest that, although urchins feeding on C. fragile are capable of surviving, growing, and reproducing, the replacement of kelp by C. fragile in some areas might negatively affect urchin populations as they continue to repopulate the shallow subtidal zone.





Similar content being viewed by others
References
Benson EE, Rutter JC, Cobb AH (1983) Seasonal variation in frond morphology and chloroplast physiology of the intertidal alga Codium fragile (Suringar) Hariot. New Phytol 95:569–580
Bird CJ, Dadswell MJ, Grund DW (1993) First record of the potential nuisance alga Codium fragile spp. tomentosoides (Chlorophyta, Caulerpales) in Atlantic Canada. Proc N S Inst Sci 40:11–17
Black R, Codd C, Hebbert D, Vink S, Burt J (1984) The functional significance of the relative size of Aristotle’s lantern in the sea urchin Echinometra mathaei (de Blainville). J Exp Mar Biol Ecol 77:81–97
Black R, Johnson MS, Trendall JT (1982) Relative size of Aristotle’s lantern in Echinometra mathaei occurring at different densities. Mar Biol 71:101–106
Brady SM, Scheibling RE (2005) Repopulation of the shallow subtidal zone by green sea urchins (Strongylocentrotus droebachiensis) following mass mortality in Nova Scotia, Canada. J Mar Biol Assoc UK 85:1511–1517
Brady SM, Scheibling RE (2006) Changes in growth and reproduction of green sea urchins, Strongylocentrotus droebachiensis (Müller), during repopulation of the shallow subtidal zone after mass mortality. J Exp Mar Biol Ecol 335:277–291
Breen PA, Mann KH (1976a) Destructive grazing of kelp by sea urchins in eastern Canada. J Fish Res Board Can 33:1278–1283
Breen PA, Mann KH (1976b) Changing lobster abundance and the destruction of kelp beds by sea urchins. Mar Biol 34:137–142
Chapman ARO (1981) Stability or sea urchin dominated barren grounds following destructive grazing of kelp in St. Margaret’s Bay, eastern Canada. Mar Biol 62:307–311
Chapman AS, Scheibling RE, Chapman ARO (2002) Species introductions and changes in marine vegetation of Atlantic Canada. In: Claudi R, Nantel P, Muckle-Jeffs E (eds) Alien invaders in Canada’s waters, wetlands, and forests. Natural Resources Canada, Canadian forest service science branch, Ottawa, pp 133–148
Cho DM, Kim DS, Lee DS, Kim HR, Pyeun JH (1995) Trace components and functional saccharides in seaweed. 1. Changes in proximate composition and trace elements according to harvest season and places. Bull Korean Fish Soc 28:49–59
Cho GY, Yoon HS, Boo SM, Yarish C (2000) Atlantic kelp species Laminaria longicruris and L. saccharina (Laminariales) are conspecific. J Phycol 36(Suppl. 3):12–13
Cruz-Rivera E, Hay ME (2000) Can quantity replace quality? Food choice, compensatory feeding, and fitness of marine mesograzers. Ecology 81:201–219
Cruz-Rivera E, Hay ME (2001) Macroalgal traits and the feeding and fitness of an herbivorous amphipod: the roles of selectivity, mixing, and compensation. Mar Ecol Prog Ser 218:249–266
Daggett TL, Pearce CM, Tingley M, Robinson SM, Chopin T (2005) Effect of prepared and macroalgal diets and seed stock source on somatic growth of juvenile green sea urchins (Strongylocentrotus droebachiensis). Aquaculture 244:263–281
de Jong-Westman M, March BE, Carefoot TH (1995) The effect of different nutrient formulations in artificial diets on gonad growth in the sea urchin Strongylocentrotus droebachiensis. Can J Zool 73:1495–1502
Duggins DO (1981) Sea urchins and kelp: the effects of short-term changes in urchin diet. Limnol Oceanogr 26:391–394
Ebert TA (1968) Growth rates of the sea urchin Strongylocentrotus purpuratus related to food availability and spine abrasion. Ecology 49:1075–1091
Ebert TA (1980) Relative growth of sea urchin jaws: an example of plastic resource allocation. Bull Mar Sci 30:467–474
Ebert TA, Dixon JD, Schroeter SC, Kalvass PE, Richmond NT, Bradbury WA, Woodby DA (1999) Growth and mortality of red sea urchins Strongylocentrotus franciscanus across a latitudinal gradient. Mar Ecol Prog Ser 190:189–209
Ebert TA Russell MP (1992) Growth and mortality estimates for red sea urchin Strongylocentrotus franciscanus from San Nicolas Island, California. Mar Ecol Prog Ser 81:31–41
Fralick RA, Mathieson AC (1972) Winter fragmentation of Codium fragile (Suringar) Hariot ssp. tomentosoides (Van Goor) silva in New England. Phycologia 11:67–70
Gagnon P, Himmelman JH, Johnson LE (2003) Algal colonization in urchin barrens: defense by association during recruitment of the brown alga Agarum cribrosum. J Exp Mar Biol Ecol 290:179–196
Gagnon P, Himmelman JH, Johnson LE (2004) Temporal variation in community interfaces: kelp-bed boundary dynamics adjacent to persistent urchin barrens. Mar Biol 144:1191–1203
Garrido CL, Barber BJ (2001) Effects of temperature and food ration on gonad growth and oogenesis of the green sea urchin, Strongylocentrotus droebachiensis. Mar Biol 138:447–456
Himmelman JH (1984) Urchin feeding and macroalgal distribution in Newfoundland, eastern Canada. Nat Can 111:337–348
Himmelman JH, Nédélec H (1990) Urchin foraging and algal survival strategies in intensely grazed communities in eastern Canada. Can J Fish Aquat Sci 47:1011–1026
Johnson CR, Mann KH (1988) Diversity, patterns of adaptation, and stability of Nova Scotian kelp beds. Ecol Monogr 58:129–154
Johnson CR, Mann KH (1982) Adaptations of Strongylocentrotus droebachiensis for survival on barren grounds in Nova Scotia. In: Lawrence JM (ed) Echinoderms: Proceedings of the international conference, Tampa Bay. A.A. Balkema, Rotterdam, pp 277–283
Keats DW, Steele DH, South GR (1984) Depth-dependent reproductive output of the green sea urchin, Strongylocentrotus droebachiensis (O.F. müller), in relation to the nature and availability of food. J Exp Mar Biol Ecol 80:77–91
Kennish R, Williams GA (1997) Feeding preferences of the herbivorous crab Grapsus albolineatus: the differential influence of algal nutrient content and morphology. Mar Ecol Prog Ser 147:87–95
Klinger TS (1982) Feeding rates of Lytechinus variegatus Lamark (Echinodermata: Echinoidea) on differing physiognomies of an artificial food on a uniform composition. In: Lawrence JM (ed) Echinoderms: Proceedings of the international conference, Tampa Bay. A.A. Balkema, Rotterdam, pp 29–32
Lamare MD, Mladenov PV (2000) Modeling somatic growth in the sea urchin Evechinus chloroticus (Echinoidea: Echinometridae). J Exp Mar Biol Ecol 243:17–43
Lamare MD, Wing SR (2001) Calorific content of New Zealand marine macroalgae. NZ J Mar Freshwater Res 35:335–341
Larson BR, Vadas RL, Keser M (1980) Feeding and nutritional ecology of the sea urchin Strongylocentrotus droebachiensis in Maine, USA. Mar Biol 59:49–62
Lauzon-Guay J-S, Scheibling R (2007) Behavior of sea urchin (Strongylocentrotus droebachiensis): food mediated aggregations and density dependant facilitation. Mar Ecol Prog Ser 329:191–204
Lawrence JM (1975) On the relationships between marine plants and sea urchins. Oceanogr Mar Biol Annu Rev 13:213–286
Lawrence JM, Lawrence AL, Watts SA (2007) Feeding, digestion and digestibility. In: Lawrence JM (ed) Edible sea urchins: biology and ecology. 2nd Edn. Elsevier Science, Amsterdam, pp 135–158
Lemire M, Himmelman JH (1996) Relation of food preference to fitness for the green sea urchin, Strongylocentrotus droebachiensis. Mar Biol 127:73–78
Levin PS, Coyer JA, Petrik R, Good TP (2002) Community-wide effects of nonindigenous species on temperate rocky reefs. Ecology 83:3182–3193
Levitan DR (1991) Skeletal changes in the test and jaws of the sea urchin Diadema antillarum in response to food limitation. Mar Biol 111:431–435
Levitan DR (1992) Community structure in times past: influence of human fishing pressure on algal-urchin interactions. Ecology 73:1597–1605
Lewis CA, Ebert TA, Boren ME (1990) Allocation of 45calcium to body components of starved and fed purple sea urchins (Strongylocentrotus purpuratus). Mar Biol 105:213–222
Lowe EF, Lawrence JM (1976) Absorption efficiencies of Lytechinus variegatus (Lamarck) (Echinodermata: Echinoidea) for selected marine plants. J Exp Mar Biol Ecol 21:223–234
Meidel SK, Scheibling RE (1998a) Annual reproductive cycle of the sea urchin Strongylocentrotus droebachiensis, in differing habitats in Nova Scotia, Canada. Mar Biol 131:461–478
Meidel SK, Scheibling RE (1998b) Differences in size and age structure of subpopulations of sea urchins (Strongylocentrotus droebachiensis) in kelp beds, barren grounds and grazing fronts in relation to growth rate and nutritional condition. In: Mooi R, Telford M (eds) Echinoderms: proceedings of the international conference, San Francisco. A.A. Balkema, Rotterdam, pp 737–742
Meidel SK, Scheibling RE (1999) Effects of food type and ration on reproductive maturation and growth of the sea urchin Strongylocentrotus droebachiensis. Mar Biol 134:155–166
Miller RJ (1985) Seaweeds, sea urchins, and lobster: a reappraisal. Can J Fish Aquat Sci 42:2061–2072
Miller RJ, Mann KH (1973) Ecological energetics of the seaweed zone in a marine bay on the Atlantic coast of Canada III. Energy transformations by sea urchins. Mar Biol 18:99–114
Minor MA, Scheibling RE (1997) Effects of food ration and feeding regime on growth and reprodution of the sea urchin Strongylocentrotus droebachiensis. Mar Biol 129:159–167
Paine RT, Vadas RL (1969) Calorific values of benthic marine algae and their postulated relation to invertebrate food preference. Mar Biol 4:79–86
Prince JS, LeBlanc WG (1992) Comparative feeding preference of Strongylocentrotus droebachiensis (Echinoidea) for the invasive seaweed Codium fragile ssp. tomentosoides (Chlorophyceae) and four other seaweeds. Mar Biol 113:159–163
Propp MV (1977) Ecology of the sea urchin Strongylocentrotus droebachiensis of the Barents Sea: metabolism and regulation of abundance. Sov J Mar Biol 3:27–37
Russell MP, Meredith RW (2000) Are natural growth lines in the skeletal structures of echinoids reliable indicators of age? A test using Strongylocentrotus droebachiensis. Invert Biol 119:410–420
Scheibling RE (1986) Increased macroalgal abundance following mass mortalities of sea urchins (Strongylocentrotus droebachiensis) along the Atlantic coast of Nova Scotia. Oecologia 68:186–198
Scheibling RE, Anthony SX (2001) Feeding, growth and reproduction of sea urchins (Strongylocentrotus droebachiensis) on single and mixed diets of kelp (Laminaria spp.) and the invasive alga Codium fragile ssp. tomentosoides. Mar Biol 139:139–146
Scheibling RE, Gagnon P (2006) Competitive interactions between the invasive green alga Codium fragile ssp. tomentosoides and native canopy-forming seaweeds in Nova Scotia (Canada). Mar Ecol Prog Ser 325:1–14
Scheibling RE, Hatcher BG (2007) The ecology of Strongylocentrotus droebachiensis. In: Lawrence JM (ed) Edible sea urchins: biology and ecology. 2nd Edn. Elsevier Science, Amsterdam, pp 353–392
Scheibling RE, Hennigar AW (1997) Recurrent outbreaks of disease in sea urchins Strongylocentrotus droebachiensis in Nova Scotia: evidence for a link with large scale meteorologic and oceanographic events. Mar Ecol Prog Ser 152:155–165
Scheibling RE, Hennigar AW, Balch T (1999) Destructive grazing, epiphytism, and disease: the dynamics of sea urchin-kelp interactions in Nova Scotia. Can J Fish Aquat Sci 56:2300–2314
Stachowicz JJ, Hay M (1999) Reduced mobility is associated with compensatory feeding and increased diet breadth of marine crabs. Mar Ecol Prog Ser 188:169–178
Sumi CBT, Scheibling RE (2005) Role of grazing by sea urchins Strongylocentrotus droebachiensis in regulating the invasive alga Codium fragile ssp. tomentosoides in Nova Scotia. Mar Ecol Prog Ser 292:203–212
Theriault C, Scheibling RE, Hatcher BG, Jones W (2006) Mapping the distribution of an invasive marine alga (Codium fragile) in optically dense coastal waters using the Compact Airborne Spectrographic Imager (CASI). Can J Remote Sens 32:315–329
Thompson RJ (1982) The relationship between food ration and reproductive effort in the green sea urchin, Strongylocentrotus droebachiensis. Oecologia 56:50–57
Vadas RL (1977) Preferential feeding: an optimization strategy in sea urchins. Ecol Monogr 47:337–371
Zar J (1999) Biostatistical analysis. Prentice Hall, Upper Saddle River
Acknowledgments
We thank Ebony Wicks, Mark Ulett, John Lindley, Allison Schmidt, Olivier D’Amours, and Meagan Saunders for their assistance with diving and lab work. Jean-Sébastien Lauzon-Guay and Marie Auger Méthé and two anonymous reviewers provided valuable comments on the manuscript. The research was funded by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada (NSERC) to RES. DAL was supported by scholarships from NSERC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R.J. Thompson.
Rights and permissions
About this article
Cite this article
Lyons, D.A., Scheibling, R.E. Differences in somatic and gonadic growth of sea urchins (Stronglyocentrotus droebachiensis) fed kelp (Laminaria longicruris) or the invasive alga Codium fragile ssp. tomentosoides are related to energy acquisition. Mar Biol 152, 285–295 (2007). https://doi.org/10.1007/s00227-007-0682-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-007-0682-4