Abstract
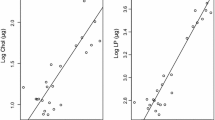
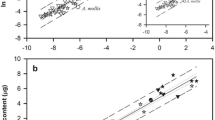
Variations in embryo size and fatty acid (FA) dynamics during embryogenesis were evaluated in deep-sea pandalids and portunid swimming crabs from the Portuguese continental margin and Madeira Island slope and compared with previous data on neritic and deep-sea lobsters and shrimps (collected between February 2001 and March 2004). Inter-specific variations in embryo size seem to be dictated primarily by phylogeny rather than by differences in reproductive or early life history traits. FA reserves were significantly correlated with embryo size (P < 0.001). Principal component analysis revealed differences among three groups (1—neritic caridean shrimps, 2—deep-sea pandalids of the genus Plesionika, and lobsters, 3—portunid crabs and the deep-sea pandalid Chlorotocus crassicornis, Costa 1871). Group 1 was clearly separated by PC1 mainly due to the higher percentage of essential C18 (linoleic and linolenic acids) and C20 (namely eicosapentaenoic) polyunsaturated FA (specific markers of primary producers). PC2 separated Group 2 from Group 3 due to differences in the percentage of several saturated FA (including odd-numbered FA—bacterial markers) and C18 monounsaturated FA (namely 18:1n − 9, a general marker of carnivory). Therefore, these differences among groups seem to result from distinctions in diet and ecological niche. Intra-specific differences in FA composition between western and southern Plesionika martia martia (A. Milne-Edwards, 1883) populations may reflect higher water temperatures on the south sub-tropical coast. Lobster embryonic development was more demanding of lipid energy than that of the other decapod species, which may reflect an evolutionary trend in decapod taxa related to an increasing degree of lecithotrophy. However, a lower FA catabolism can be interpreted as an enhanced independence of the newly hatched larvae from external energy sources. Higher FA content at hatching and, as a consequence, a greater independence from the external environment should increase the chances of larval survival.






Similar content being viewed by others
References
Ambar I, Howe MR (1979) Observations of the Mediterranean outflow. I. Mixing in the Mediterranean outflow. Deep Sea Res 26A:535–554
Anger K (2001) The biology of decapod crustacean larvae. In: Vonk R (ed) Crustacean issues, vol 14. A.A. Balkema Publishers, Lisse, 419 pp
Anger K, Harms J (1990) Elemental (CHN) and proximate biochemical composition of decapod crustacean larvae. Comp Biochem Physiol 97B:69–80
Anger K, Moreira GS (1998) Morphometric and reproductive traits of tropical caridean shrimps. J Crust Biol 18:823–838
Anger K, Moreira GS, Ismael D (2002) Comparative size, biomass, chemical composition (C, N, H) and energy concentration of caridean shrimp eggs. Invertebr Reprod Dev 32:83–93
Anger K, Lovrich GA, Thatje S, Calcagno JA (2004) Larval and early juvenile development of Lithodes santolla (Molina, 1782) (Decapoda: Anomura: Lithodidae) reared at different temperatures in the laboratory. J Exp Mar Biol Ecol 306:217–230
Auel H, Harjes M, da Rocha R, Stübing D, Hagen W (2002) Lipid biomarkers indicate different ecological niches and trophic relationships of the Arctic hyperiid amphipods Themisto abyssorum and T. libellula. Polar Biol 25:374–383
Barnich R (1996) The larvae of the Crustacea Decapoda (excl. Brachyura) in the plankton of the French Mediterranean coast (identification keys and systematic review). Cuvillier, Göttingen
Bell MV, Dick JR (1990) The fatty acid composition of phospholipids from the eyes of the northern deepwater prawn Pandalus borealis. Biochem Soc Trans 18:907–908
Biscoito MJ (1993) An account of the shrimps of the family Pandalidae (Crustacea, Decapoda, Caridea) in Madeiran waters. Courier Forschunginstitut Senckenberg 159:321–325
Boddeke R (1982) The occurrence of winter and summer eggs in the brown shrimp (Crangon crangon) and the pattern of recruitment. Neth J Sea Res 16:151–162
Calado R, Narciso L (2000) Camarões e Lagostas da Costa Continental Portuguesa. Prémio do Mar Rei D. Carlos—6ª Edição. Câmara Municipal de Cascais, Cascais, Portugal
Calado R, Bartilotti C, Narciso L, dos Santos A (2004) Redescription of the larval stages of Lysmata seticaudata (Risso, 1816) (Crustacea, Decapoda, Hippolytidae) reared under laboratory conditions. J Plankton Res 26:737–752
Calado R, Rosa R, Nunes ML, Narciso L (2005) Amino and fatty acid dynamics of Lysmata seticaudata (Decapoda: Hippolytidae) embryos during early and late reproductive season. Mar Biol 147:341–351
Cartes JE (1993) Diets of deep-water pandalid shrimps on the Western Mediterranean slope. Mar Ecol Prog Ser 96:49–61
Cartes JE, Abelló P, Lloris D, Carbonell A, Torres P, Maynou F, De Sola LG (2002) Feeding guilds of western Mediterranean demersal fish and crustaceans: an analysis based on a spring survey. Sci Mar 66(Suppl. 2):209–220
Charmantier G, Charmantier-Daures M (2001) Ontogeny of osmoregulation in crustaceans: the embryonic phase. Am Zool 41:1078–1089
Clarke A (2003) Costs and consequences of evolutionary temperature adaptation. TREE 18 (11):573–581
Cohen Z, Von Shak A, Richmond A (1988) Effect of environmental conditions on fatty acid composition of the red algae Porphyridium cruentum: correlation to growth rate. J Phycol 24:328–332
Company JB, Sardà F (1998) Metabolic rates and energy content of deep-sea benthic decapod crustaceans in the Western Mediterranean Sea. Deep Sea Res I 45:1861–1880
Cristo M, Cartes JE (1998) A comparative study of the feeding ecology of Nephrops norvegicus (L.) (Decapoda: Nephropidae) in the bathyal Mediterranean and the adjacent Atlantic. Sci Mar 62:81–90
Dalsgaard J, St. John M, Kattner G, Müller-Navarra D, Hagen W (2003) Fatty acid trophic markers in the pelagic marine environment. Adv Mar Biol 46:225–340
Della Croce N (1961) Considerazioni su Polybius henslowii, Leach (Crustacea, Brachyura). Boll Museo Ist Biol, Genoa, 31:1–13
Evjemo O, Danielsen T, Olsen Y (2001) Losses of lipid, protein and n − 3 fatty acids in enriched Artemia franciscana starved at different temperatures. Aquaculture 193:65–80
Farmer ASD (1975) Reproduction in Nephrops norvegicus (Decapoda: Nephropidae). J Zool 174:161–183
Fiúza AFG, Hamann M, Ambar I, Del Rio GD, González N, Cabanas JM (1998) Water masses and their circulation off western Iberia during May 1993. Deep Sea Res I 45:1127–1160
Fox C, Brown JH, Briggs M (1994) The nutrition of prawns and shrimp in aquaculture—a review of recent research. In: Muir JF, Roberts RJ (eds) Recent advances in aquaculture V. Blackwell Science, Oxford, pp 131–206
Gage JD, Tyler PA (1991) Deep-sea biology: a natural history of organisms at the deep-sea floor. Cambridge University Press, London
García-Guerrero M, Villarreal MH, Racotta IS (2003) Effect of temperature on lipids, proteins, and carbohydrates levels during development from egg extrusion to juvenile stage of Cherax quadricarinatus (Decapoda: Parastacidae). Comp Biochem Physiol A 135:147–154
González JA, Tuset VM, Lozano IJ, Santana JI (1997) Biology of Plesionika narval (Crustacea, Decapoda, Pandalidae) around the Canary Islands (Eastern Central Atlantic). Estuar Coast Shelf Sci 44:339–350
Herring PJ (1973) Depth distribution of the carotenoid pigments and lipids of some oceanic animals. 2. Decapod crustaceans. J Mar Biol Assoc UK 53:539–562
Herring PJ (1974) Size, density and lipid content of some decapod eggs. Deep Sea Res 21:91–94
Kattner G, Wehrtmann IS, Merck T (1994) Interannual variations of lipids and fatty acids during larval development of Crangon spp. in the German Bight, North Sea. Comp Biochem Physiol B 107:103–110
Kattner G, Graeve M, Calcagno JA, Lovrich GA, Thatje S, Anger K (2003) Lipid, fatty acid and protein utilization during lecithotrophic larval development of Lithodes santolla (Molina) and Paralomis granulosa (Jacquinot). J Exp Mar Biol Ecol 292:61–74
King MG, Butler AJ (1985) Relationship of life-history patterns to depth in deep-water caridean shrimps (Crustacea: Natantia). Mar Biol 86:129–138
Labropoulou M, Kostikas I (1999) Patterns of resource use in deep-water decapods. Mar Ecol Prog Ser 184:171–182
Lagardère J (1971) Les crevettes des côtes du Maroc. Travaux de l´’Institut Scientifique Cherifien et de la Faculte des Sciences, Rabat, série zoologique 36:1–140
Lardies MA, Wehrtmann IS (2001) Latitudinal variation in the reproductive biology of Betaeus truncatus (Decapoda: Alpheidae) along the Chilean coast. Ophelia 55:55–67
Lee RF (1991) Lipoproteins from the hemolymph and ovaries of marine invertebrates. Adv Comp Environ Physiol 7:187–207
Lee RF, Hagen W, Kattner G (2006) Lipid storage in marine zooplankton. Mar Ecol Prog Ser 307:273–306
Lepage G, Roy CC (1986) Direct transesterification of all classes of lipids in one-step reaction. J Lipid Res 27:114–119
Los DA, Murata N (2004) Membrane fluidity and its role in the perception of environmental signals. Biochem Biophys Acta 1666:142–157
McCullagh P, Nelder JA (1989) Generalized linear models. Chapman & Hall, London
Morais S, Narciso L, Calado R, Nunes ML, Rosa R (2002) Lipid dynamics during the embryonic development of Plesionika martia martia (Decapoda; Pandalidae), Palaemon serratus and Palaemon elegans (Decapoda; Palaemonidae): relation to metabolic consumption. Mar Ecol Prog Ser 242:195–204
Morley SA, Belchier M, Dickson J, Mulvey T (2006) Reproductive strategies of sub-Antarctic lithodid crabs vary with habitat depth. Mar Biol 29:581–584
Munro D, Thomas DW (2004) The role of polyunsaturated fatty acids in the expression of torpor by mammals: a review. Zoology 107:29–48
Narciso L (1999) Aquaculture development: perspectives for the next decade. In: Beurier J-P, Kiss A, Mahmoudi S (eds) New technologies and law of the marine environment. Kluwer Law International, London, pp 41–52
Omori M (1974) The biology of pelagic shrimps in the oceans. Adv Mar Biol 12:233–324
Orav-Kotta H (2004) Habitat choice and feeding activity of benthic suspension feeders and mesograzers in the Northern Baltic Sea. Ph.D. thesis, Universitatis Tartuensis, 117 p
Porter ML, Pérez-Losada M, Crandall KA (2005) Model-based multi-locus estimation of decapod phylogeny and divergence times. Mol Phylogenet Evol 37:355–369
Rainuzzo JR, Reitan KI, Olsen Y (1997) The significance of lipids at early stages of marine fish: a review. Aquaculture 155:103–116
Rosa R, Nunes ML (2002) Biochemical changes during the reproductive cycle of deep-sea decapod Nephrops norvegicus on the south Portuguese coast. Mar Biol 141(6):1001–1009
Rosa R, Nunes ML (2003a) Tissue biochemical composition in relation to the reproductive cycle of deep-sea decapod Aristeus antennatus in the south Portuguese coast. J Mar Biol Assoc UK 83:963–970
Rosa R, Nunes ML (2003b) Biochemical composition of deep-sea decapod crustaceans with two different benthic life strategies in the Portuguese south coast. Deep Sea Res I 50(1):119–130
Rosa R, Morais S, Calado R, Narciso L, Nunes ML (2003) Biochemical changes during the embryonic development of Norway lobster, Nephrops norvegicus (Crustacea: Decapoda). Aquaculture 221(1–4):507–522
Rosa R, Calado R, Andrade AM, Narciso L, Nunes ML (2005) Changes in amino acids and lipids during embryogenesis of European lobster, Homarus gammarus (Crustacea: Decapoda). Comp Biochem Physiol B 140:241–249
dos Santos A (1999) Larvas de Crustáceos Decápodes ao Largo da Costa Portuguesa. Ph.D. thesis, University of Lisbon, Lisbon, Portugal
Sargent JR (1995) Origins and functions of egg lipids: nutritional implications. In: Bromage NR, Roberts RJ (eds) Broodstock management and egg and larval quality. Blackwell Science, Oxford, pp 353–372
Sargent JR, Henderson RJ, Tocher DR (1989) The lipids. In: Halver JE (ed) Fish nutrition. Academic, New York, pp 153–218
Scott C, Kwasniewski S, Falk-Petersen S, Sargent JR (2002) Species differences, origins and functions of fatty alcohols and fatty acids in the wax esters and phospholipids of Calanus hyperboreus, C. glacialis and C. finmarchicus from Arctic waters. Mar Ecol Prog Ser 235:127–134
Teshima S-I (1997) Phospholipids and sterols. In: D’Abramo LR, Conklin DE, Akiyama DM (eds) Crustacean nutrition. World Aquaculture Society, Baton Rouge, pp 85–107
Thatje S, Schnack-Schiel S, Arntz WE (2003) Developmental trade-offs in Subantarctic meroplankton communities and the enigma of low decapod diversity in high southern latitudes. Mar Ecol Prog Ser 260:195–207
Thatje S, Lovrich GA, Anger K (2004) Egg production, hatching rates, and abbreviated larval development of Campylonotus vegans Bate, 1888 (Crustacea: Decapoda: Caridea), in Subantarctic waters. J Exp Mar Biol Ecol 301:15–27
Thatje S, Anger K, Calcagno JA, Lovrich GA, Portner HO, Arntz WE (2005) Challenging the cold: crabs reconquer the Antarctic. Ecology 86(3):619–625
Turner RL, Lawrence JM (1979) Volume and composition of echinoderm eggs: implications for the use of egg size in life-history models. In: Stancyk SE (eds) Reproductive ecology of marine invertebrates. University of South Carolina Press, Columbia, pp 25–40
Udekem d’Acoz C (1999) Inventaire et distribution des crustacés décapodes de l’Atlantique nord-oriental, de la Méditerranée et des eaux continentales adjacentes au nord de 25°N. Patrimoines naturels (M.N.H.N./S.P.N.) 40:1–383
Volkman JK, Barrett SM, Blackburn SI, Mansour MP, Sikes EL, Gelin F (1998) Microalgal biomarkers: a review of recent research developments. Org Geochem 29:1163–1179
Wehrtmann IS, Graeve M (1998) Lipid composition and utilization in developing eggs of two tropical marine caridean shrimps (Decapoda: Caridea: Alpheidae: Palaemonidae). Comp Biochem Physiol B 121:457–463
Wenner AM, Kuris A (1991) Crustacean egg production. Crustacean issues, vol 7. A.A. Balkema, Rotterdam
Winberg GC (1971) Methods for estimation of production of aquatic animals. Academic, New York
Zariquiey Alvarez R (1968) Crustáceos Decápodos Ibéricos. Inv Pesq 32:1–510
Acknowledgments
The Foundation for Science and Technology supported this study through a doctoral grant to the first author and also through the research project POCTI/BSE/43340/2001. Gratitude is due to Dr Ricardo Araújo and Dr Manuel Biscoito from the Estação de Biologia Marinha do Funchal—Museu Municipal do Funchal (Historia Natural) for their help obtaining the Madeira specimens. The authors would also like to thank S. Morais, A. Rodrigues, C. Pires and T. Pimentel for their support during field and laboratory work and Dr Brad Seibel for critically reading and editing the English text. The experiments described comply with current Portuguese and EU laws.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.P. Grassle, New Brunswick.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Rosa, R., Calado, R., Narciso, L. et al. Embryogenesis of decapod crustaceans with different life history traits, feeding ecologies and habitats: a fatty acid approach. Mar Biol 151, 935–947 (2007). https://doi.org/10.1007/s00227-006-0535-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-006-0535-6