Abstract
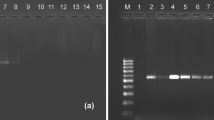
The polymerase chain reaction (PCR) offers a sensitive and selective way to detect trace amounts of biological remnants. Here, we show that this simple molecular technique can be applied to identify prey copepods in the fecal pellets of carnivorous zooplankton. Using variation in the mitochondrial cytochrome C oxidase subunit I (mtCOI) sequence, we developed a species-specific oligonucleotide PCR primer (COI-2026) for Calanus helgolandicus. In a “touch-down” PCR, Calanus DNA was amplified from pellets collected from freshly incubated individuals of the carnivorous copepod Pareuchaeta norvegica. Positive results could easily be detected by agarose gel electrophoresis.



Similar content being viewed by others
References
Andrews RM, Kubacka I, Chinnery PF, Lightowlers RN, Turnbull DM, Howell N (1999) Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat Genet 23:147
Asahida T, Yamashita Y, Kobayashi T (1997) Identification of consumed stone flounder, Kareius bicoloratus (Basilewsky), from the stomach contents of sand shrimp, Crangon affinis (De Haan) using mitochondrial DNA analysis. J Exp Mar Biol Ecol 217:153–163
Bagøien E, Kaartvedt S, Øverås S (2000) Seasonal vertical migrations of Calanus spp. in Oslofjorden. Sarsia 85:299–311
Bucklin A, Guarnieri M, Hill RS, Bentley AM, Kaartvedt S (1999) Taxonomic and systematic assessment of planktonic copepods using mitochondrial COI sequence variation and competitive, species-specific PCR. Hydrobiologia 401:239–254
Clary DO, Wolstenholme DR (1985) The mitochondrial-DNA-molecule of Drosophila yakuba-nucleotide-sequence, gene organization, and genetic code. J Mol Evol 22:252–271
Dennis C (2003) Error reports threaten to unravel databases of mitochondrial DNA. Nature 421:773–774
Fleddum A, Kaartvedt S, Ellertsen B (2001) Distribution and feeding of the carnivorous copepod Paraeuchaeta norvegica in habitats of shallow prey assemblages and midnight sun. Mar Biol 139:719–726
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I form diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Forster P (2003) To err is human. Ann Hum Genet 67:2–4
Fowler SW, Small LF (1972) Sinking rates of euphausiid fecal pellets. Limnol Oceanogr 17:293–296
Gauld DT (1957) Peritrophic membrane in calanoid copepods. Nature 179:325–326
Greene CH, Landry MR (1985) Patterns of prey selection in the cruising calanoid predator Euchaeta elongata. Ecology 66:1408–1416
Hare MP, Palumbi SR, Butman CA (2000) Single-step species identification of bivalve larvae using multiplex polymerase chain reaction. Mar Biol 137:953–961
Hill RS, Allen LD, Bucklin A (2001) Multiplexed species-specific PCR protocol to discriminate four N. Atlantic Calanus species, with an mtCOI gene tree for ten Calanus species. Mar Biol 139:279–287
Kaartvedt S, Larsen T, Hjelmseth K, Onsrud MSR (2002) Is the omnivorous krill Meganyctiphanes norvegica primarily a selectively feeding carnivore? Mar Ecol Prog Ser 228:193–204
Nazarenko IA, Bhatnagar SK, Hohman RJ (1997) A closed tube format for amplification and detection of DNA based on energy transfer. Nucleic Acids Res 25:2516–2521
Nejstgaard JC, Frischer ME, Raule CL, Gruebel R, Kohlberg KE, Verity PG (2003) Molecular detection of algal prey in copepod guts and fecal pellets. Limnol Oceanogr Meth 1:29–38
Nott JA, Corner EDS, Mavin LJ, Ohara SCM (1985) Cyclical contributions of the digestive epithelium to fecal pellet formation by the copepod Calanus helgolandicus. Mar Biol 89:271–279
Øresland V, Ward P (1993) Summer and winter diet of 4 carnivorous copepod species around South-Georgia. Mar Ecol Prog Ser 98:73–78
Palumbi SR (1996) Nucleic acids II: the polymerase chain reaction. In: Hillis DM, Moritz C, Mable BK (eds) Molecular systematics, 2nd edn. Sinauer, Sunderland, Mass., USA, p 655
Rudi K, Skulberg OM, Skulberg R, Jakobsen KS (2000) Application of sequence-specific labeled 16S rRNA gene oligonucleotide probes for genetic profiling of cyanobacterial abundance and diversity by array hybridization. Appl Environ Microbiol 66:4004–4011
Sambrook J, Russell DW (2001) Protocol 2: detection of DNA in agarose gels molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y., USA
Schofield P, Pell AN, Krause DO (1997) Molecular beacons: trial of a fluorescence-based solution hybridization technique for ecological studies with ruminal bacteria. Appl Environ Microbiol 63:1143–1147
Suzuki H, Tamate BH, Sasaki H (2000) Identification of fecal pellet producers through copepod-derived DNA sequence. Bull Plankton Soc Jpn 47:136–139
Suzuki H, Sasaki H, Fukuchi M (2003) Loss processes of sinking fecal pellets of zooplankton in the mesopelagic layers of the Antarctic marginal ice zone. J Oceanogr 59:809–818
Symondson WOC (2002) Molecular identification of prey in predator diets. Mol Ecol 11:627–641
Tiselius P, Jonsson PR (1997) Effects of copepod foraging behavior on predation risk: an experimental study of the predatory copepod Pareuchaeta norvegica feeding on Acartia clausi and A. tonsa (Copepoda). Limnol Oceanogr 42:164–170
Torgersen T (2001) Visual predation by the euphausiid Meganyctiphanes norvegica. Mar Ecol Prog Ser 209:295–299
Vestheim H, Edvardsen B, Kaartvedt S (2005) State-dependent vertical distribution of the carnivore copepod Pareuchaeta norvegica. J Plankton Res 27:19–26
Yen J (1985) Selective predation by the carnivorous marine copepod Euchaeta elongata—laboratory measurements of predation rates verified by field observations of temporal and spatial feeding patterns. Limnol Oceanogr 30:577–597
Acknowledgements
This study was supported by the Norwegian Research Council (project 140286/120).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kühl, Helsingør
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Vestheim, H., Edvardsen, B. & Kaartvedt, S. Assessing feeding of a carnivorous copepod using species-specific PCR. Marine Biology 147, 381–385 (2005). https://doi.org/10.1007/s00227-005-1590-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-1590-0