Abstract
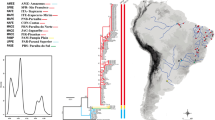
Partial sequences of the mitochondrial DNA (mtDNA) gene cytochrome oxidase subunit 1 (COI) were analysed from individuals of the coralline demosponge Astrosclera willeyana sensu lato out of ten Indo-Pacific populations from the Red Sea to the central Pacific. This taxon is widely distributed in cryptic coral reef habitats of the Indo-Pacific and is regarded as a modern representative of long-extinct, formerly reef-building stromatoporoid-type sponges. The aims were to clarify phylogeographic and taxonomic relationships in this “living fossil” and to explore mitochondrial DNA sequence variation over a wide geographic range. Very low variability was observed across the Indo-Pacific, as only three COI haplotypes were identified, with a maximum p-distance of 0.418% and low nucleotide diversity (π=0.00049). Very low genetic structure was revealed among populations: Haplotype 1 was found in all specimens from nine Pacific populations (N=45), separated by distances of more than 7,000 km; haplotype 2 was restricted to the Red Sea population (N=4); haplotype 3 was only found in the Tuamoto specimens (N=7). COI data presented here do not support the hypothesis of at least two sibling species belonging to genus Astrosclera in the Pacific. Considering the maximum geographic distance of more than 20,000 km between sampled populations, mtDNA COI sequence variation observed here is among the lowest reported to date for a diploblastic taxon and adds to the growing evidence of a general mtDNA conservation in sponges. It is argued that this low mtDNA variation in A. willeyana s.l. is due to a low rate of mtDNA evolution caused by a combination of long generation time and low metabolic rate.

Similar content being viewed by others
Notes
The concept of an aggregate of sibling species within Recent genus Astrosclera is followed here by adding sensu lato (= in the broad sense) to the specific name and sensu stricto (= in the strict sense) when referring to individuals from populations in the vicinity of the type locality Lifou (New Caledonia) (Lister 1900).
References
Aerts LAM (2000) Dynamics behind standoff interactions in three reef sponge species and the coral Montastraea cavernosa. Mar Ecol 21:191–204
Avise J (2000) Phylogeography: the history and formation of species. Harvard University Press, Cambridge
Avise JC (1994) Molecular markers, natural history and evolution. Chapman & Hall, New York
Bailey WJ, Fitch DH, Tagle DA, Czelusniak J, Slightom JL, Goodman M (1991) Molecular evolution of the psi eta-globin gene locus: gibbon phylogeny and the hominoid slowdown. Mol Biol Evol 8:155–184
Birky CWJ, Maruyama T, Fuerst P (1983) An approach to population and evolutionary genetic theory for genes in mitochondria and chloroplasts, and some results. Genetics 103:513–527
Boore JL (1999) Animal mitochondrial genomes. Nucleic Acids Res 27:1767–1780
Boyle JS, Lew AM (1995) An inexpensive alternative to glassmilk for DNA purification. Trends Genet 11:8
Brown WM, George MJ, Wilson AC (1979) Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci USA 76:1967–1971
Diaz MC, Rutzler K (2001) Sponges: an essential component of Caribbean coral reefs. Bull Mar Sci 69:535–546
Duran S, Pascual M, Turon X (2003) Low levels of genetic variation in mtDNA sequences over the western Mediterranean and Atlantic range of the sponge Crambe crambe (Poecilosclerida). Mar Biol 144:31–35
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome C oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Lavrov DV, Forget L, Kelly M, Lang BF (2005) Mitochondrial genomes of two demosponges provide insights into an early stage of animal evolution. Mol Biol Evol 22:1231–1239
Lavrov DV, Lang BF (2005) Transfer RNA gene recruitment in mitochondrial DNA. Trends Genet 21:129–133
Lister JJ (1900) Astrosclera willeyana, the type of a new family of sponges. Zool Results 4:461–482
Martin AP, Palumbi SR (1993) Body size, metabolic rate, generation time, and the molecular clock. Proc Natl Acad Sci USA 90:4087–4091
Moritz C, Dowling TE, Brown WM (1987) Evolution of the animal mitochondrial DNA: relevance for population biology and systematics. Annu Rev Ecol Syst 18:269–292
Pont-Kingdon GA, Okada NA, Macfarlane JL, Beagley CT, Wolstenholme DR, Cavalier Smith T, Clark Walker GD (1995) A coral mitochondrial mutS gene. Nature 375:109–111
Quesada H, Warren M, Skibinski DOF (1998) Nonneutral evolution and differential mutation rate of gender-associated mitochondrial DNA lineages in the marine mussel Mytilus. Genetics 149:1511–1526
Rambaut A (1996) Se-Al: Sequence Alignment Editor. available at: http://evolve.zoo.ox.ac.uk/
Reitner J, Wörheide G, Thiel V, Gautret P (1996) Reef caves and cryptic habitats of Indo-Pacific reefs; distribution patterns of coralline sponges and microbialites. Global and regional controls on biogenic sedimentation; 1: reef evolution, research reports. Göttinger Arbeiten zur Geologie und Palaeontologie Sb2:91–100
Rozas J, Rozas R (1999) DnaSP version 3: an integrated program for molecular population genetics and molecular evolution analysis. Bioinformatics 15:174–175
Shearer T, van Oppen MJH, Romano SL, Wörheide G (2002) Slow mitochondrial DNA sequence evolution in the Anthozoa (Cnidaria). Mol Ecol 11:2475–2487
Swofford DL (1998) PAUP*. Phylogenetic analysis using parsimony (*and other methods), version 4. Sinauer Associates, Sunderland
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tsaousis AD, Martin DP Ladoukakis ED, Posada D, Zouros E (2005) Widespread recombination in published animal mtDNA sequences. Mol Biol Evol 22:925–933
Uriz MJ, Maldonado M, Turon X, Marti R (1998) How do reproductive output, larval behaviour, and recruitment contribute to adult spatial patterns in Mediterranean encrusting sponges? Mar Ecol Prog Ser 167:137–148
Vacelet J (1981) Éponges hypercalcifiées (Pharétronides, Sclérosponges) des cavités des récifs coralliens de Nouvelle-Calédonie. Bull Mus Nat Hist Natur (Paris), Sec A 3:313–351
Watkins RF, Beckenbach AT (1999) Partial sequence of a sponge mitochondrial genome reveals sequence similarity to Cnidaria in Cytochrome Oxidase Subunit II and the large ribosomal RNA subunit. J Mol Evol 48:542–554
Wood R (1987) Biology and revised systematics of some late Mesozoic stromatoporoids. Spec Pap Palaeontol 37:1–89
Wörheide G, Degnan BM, Hooper JNA, Reitner J (2002) Phylogeography and taxonomy of the Indo-Pacific reef cave dwelling coralline demosponge Astrosclera willeyana - new data from nuclear ITS sequences. In: Moosa KM, Soemodihardjo S, Soegiarto A, Romimohtarto K, Nontji A, Soekarno Suharsono (eds) Proceedings of the 9th International coral reef symposium, vol 1. Ministry for Environment, Indonesian Institute of Sciences, International Society for Reef Studies, Jakarta, pp 339–346
Wörheide G, Degnan BM, Hooper JNA (2000) Population phylogenetics of the common coral reef sponges Leucetta spp. and Pericharax spp. (Porifera: Calcarea) from the Great Barrier Reef and Vanuatu. Abstracts, 9th international coral reef symposium, Bali, 23 October 2000
Wörheide G, Hooper JNA, Degnan BM (2002) Phylogeography of western Pacific Leucetta ‘chagosensis’ (Porifera: Calcarea) from ribosomal DNA sequences: implications for population history and conservation of the Great Barrier Reef World Heritage Area (Australia). Mol Ecol 11:1753–1768
Wörheide G (1998) The reef cave dwelling ultraconservative coralline demosponge Astrosclera willeyana Lister 1900 from the Indo-Pacific. Micromorphology, ultrastructure, biocalcification, isotope record, taxonomy, biogeography, phylogeny. Facies 38:1–88
Yue GH, Orban L (2001) Rapid isolation of DNA from fresh and preserved fish scales for polymerase chain reaction. Mar Biotechnol 3:199–204
Acknowledgements
Funding from the BMB+F Juniorprofessor-Programme and from the Deutsche Forschungsgemeinschaft (DFG) is highly acknowledged. I wish to thank Dr. John Hooper (Brisbane), Dr. Gustav Paulay (Gainesville, FL) and Dr. Tomoki Kase (Tokyo) for their help in obtaining samples, Laura Epp, Luciana Macis and Martin Dohrmann for assistance in the lab, and Dr. Dirk Erpenbeck, Oliver Voigt (both Department of Geobiology, Göttingen) and two anonymous reviewers for valuable comments on earlier drafts of this manuscript. I would also like to thank Fernando Parra (Universidad Nacional de Colombia) for sharing his unpublished results on COI variation in the genus Agelas. I would like to thank the Egyptian Environmental Affairs Agency (EEAA) for permission to carry out field work in Egypt and the staff of the Red Sea Environmental Centre (RSEC) for their support. All experiments carried out comply with current German laws.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Kinne, Oldendorf/Luhe
Rights and permissions
About this article
Cite this article
Wörheide, G. Low variation in partial cytochrome oxidase subunit I (COI) mitochondrial sequences in the coralline demosponge Astrosclera willeyana across the Indo-Pacific. Marine Biology 148, 907–912 (2006). https://doi.org/10.1007/s00227-005-0134-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-0134-y