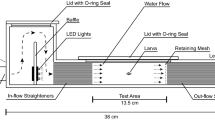
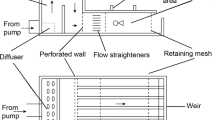
Abstract
The swimming abilities of larval fishes are important for their survival, potentially affecting their ability to avoid predators, obtain food and control dispersal patterns. Near settlement swimming abilities may also influence spatial and temporal patterns of recruitment. We examined Critical speed (U-crit) swimming ability in late stage larvae of 89 species of coral reef fishes from the Great Barrier Reef and the Caribbean. Coefficients of variation in U-crit calculated at the individual level were high (28.4%), and this was not explained by differences in size or condition factor of these same larvae. Among species U-crit ranged from 5.5 cm s−1 to 100.8 cm s−1 (mean=37.3 cm s−1), with 95% of species able to swim faster than the average current speed around Lizard Island, suggesting that most species should be capable of influencing their spatial and temporal patterns of settlement. Inter-specific differences in swimming ability (at both the family and species levels) were significantly correlated with size and larval morphology. Correlations were found between swimming performance and propulsive area, fineness ratio and aspect ratio, and these morphological parameters may prove useful for predicting swimming ability in other taxa. Overall, the swimming speeds of larvae from the same families at the two locations were relatively similar, although the Lutjanidae and Acanthuridae from the Caribbean were significantly slower than those from the great barrier reef. Differences in swimming speed and body form among late stage larvae suggests that they will respond differently to factors influencing survival and transport during their pelagic phase, as well as habitat use following settlement.





Similar content being viewed by others
References
Bainbridge R (1960) Speed and stamina in three fish. J Exp Biol 370:129–153
Bellwood DR, Fisher R (2001) Relative swimming speeds in reef fish larvae. Mar Ecol Prog Ser 211:299–303
Boily P, Magnan P (2002) Relationship between individual variation in morphological characters and swimming costs in brook charr (Salvelinus fontinalis) and yellow perch (Perca falvescens). J Exp Biol 205:1031–1036
Botsford L, Hastings A, Gaines S (2001) Dependence of sustainability on the configuration of marine reserves and larval dispersal distance. Ecol Lett 4:144–150
Brett JR (1964) The respiratory metabolism and swimming performance of young sockeye salmon. J Fish Res Bd Can 21:1183–1226
Drucker EG (1996) The use of gait transition speed in comparative studies of fish locomotion. Am Zool 36:555–566
Farlinger S, Beamish FW (1977) Effects of time and velocity increments on the critical swimming speed of largemouth bass (Micropterus salmoides). Trans Am Fish Soc 106:436–439
Farrell AP, Johansen JA, Suarez RK (1991) Effects of exercise-training on cardiac performance and muscle enzymes in rainbow trout, Oncorhynchus mykiss. Fish Physiol Biochem 9:303–312
Fisher R (2005) Swimming speeds of larval coral reef fishes: impacts on self-recruitment and dispersal. Mar Ecol Prog Ser 285:223–232
Fisher R, Bellwood DR (2002) The influence of swimming speed on sustained swimming performance of late-stage reef fish larvae. Mar Biol 140:801–807
Fisher R, Bellwood DR (2003) Undisturbed swimming behaviour and nocturnal activity of coral reef fish larvae. Mar Ecol Prog Ser 263:177–188
Fisher R, Wilson SK (2004) Maximum sustainable swimming speeds of nine species of late stage larval reef fishes. J Exp Mar Biol Ecol 312:171–186
Fisher R, Bellwood DR, Job SD (2000) The development of swimming abilities in reef fish larvae. Mar Ecol Prog Ser 202:163–173
Frith CA, Leis JM, Goldman B (1986) Currents in the Lizard island region of the great barrier reef lagoon and their relevance to potential movements of larvae. Coral Reefs 5:81–92
Fulton CJ, Bellwood DR (2004) Wave exposure, swimming performance, and the structure of tropical and temperate reef fish assemblages. Mar Biol 144:429–437
Fulton CJ, Bellwood DR, Wainwright PC (2001) The relationship between swimming ability and habitat use in wrasses (Labridae). Mar Biol 139:25–33
Green B, Fisher R (2004) Temperature influences swimming speed, growth and larval duration in coral reef fish larvae. J Exp Mar Biol Ecol 299:115–132
Hartwell SI, Otto RG (1978) Swimming performance of juvenile menhaden (Brevoortia tyrannus). Trans Am Fish Soc 107:793–798
Hartwell SI, Otto RG (1991) Critical swimming capacity of the Atlantic Silverside, Menidia menidia L. Estuaries 14:218–221
Hawkins DK, Quinn TP (1996) Critical swimming velocity and associated morphology of juvenile coastal cutthroat trout (Onchorhynchus clarkii), steelhead trout (Oncorhynchus mykiss), and their hybrids. Can J Fish Aquat Sci 53:1487–1496
Hunter J (1981) Feeding Ecology and predation of marine fish larvae. In: Lasker R (ed) Marine fish larvae. University of Washington, Washington, pp 33–79
Jones DR, Kiceniuk JW, Bamford OS (1974) Evaluation of the swimming performance of several fish species from Mackenzie River. J Fish Res Bd Can 31:1641–1647
Kerrigan BA (1996) Temporal patterns in size and condition at settlement in two tropical reef fishes (Pomacentridae: Pomacentrus amionensisandP. nagasakiensis). Mar Ecol Prog Ser 135:27–41
Kolok AS (1991) Photoperiod alters the critical swimming speed of juvenile largemouth bass, Micropterus salmoides, acclimated to cold water. Copeia 1991:1085–1090
Kolok AS (1999) Interindividual variation in the prolonged locomotor performance of ectothermic vertebrates: a comparison of fish and herpetofaunal methodologies and a brief review of the recent fish literature. Can J Fish Aquat Sci 56:700–710
Kolok AS, Oris JT (1995) The relationship between specific growth rate and swimming performance in male fathead minnows (Pimephales promelas). Can J Zool/Rev Can Zool 73:2165–2167
Koumoundouros G, Sfakianakis DG, Divanach P, Kentouri M (2002) Effect of temperature on swimming performance of sea bass juveniles. J Fish Biol 60:923–932
Leis JM, Carson-Ewart BM (1997) In situ swimming speeds of the late pelagic larvae of some Indo-Pacific coral-reef fishes. Mar Ecol Prog Ser 159:165–174
Leis JM, Carson-Ewart BM (2000) Larvae of Indo-Pacific coastal fishes: an identification guide to marine fish larvae. Brill, Leiden
Leis JM, Fisher R (2005) Swimming speed of late-stage reef-fish larvae measured in the laboratory and in the field: a comparison of critical speed and in situ speed. In: Proceedings of the 10th international coral reef symposium, Okinawa (in Press)
Lowe CG (1996) Kinematics and critical swimming speed of juvenile scalloped hammerhead sharks. J Exp Biol 199:2605–2610
MacKenzie BR, Kiorboe T (1995) Encounter rates and swimming behavior of pause-travel and cruise larval fish predators in calm and turbulent laboratory environments. Limnol Oceanogr 40:1278–1289
McCormick M (1998) Condition and growth of reef fish at settlement: is it important? Aust J Ecol 23:258–264
Miller T, Crowder LB, Rice JA, Marschall EA (1988) Larval size and recruitment mechanisms in fishes: toward a conceptual framework. Can J Fish Aquat Sci 45:1657–1670
Montgomery JC, Tolimieri N, Haine O (2001) Active habitat selection by pre-settlement reef fishes. Fish Fish 2:261–277
Mora C, Sale PF (2002) Are populations of coral reef fish open or closed?. TREE 17:422–428
Myrick CA, Cech JJ, (2000) Swimming performance of four California stream fishes: temperature effects. Environ Biol Fish 58:289–295
Peake S, McKinley RS (1998) A re-evaluation of swimming performance in juvenile salmonids relative to downstream migration. Can J Fish Aquat Sci 55:682–687
Plaut I (2000) Effects of fin size on swimming performance, swimming behaviour and routine activity of zebrafish Danio rerio. J Exp Biol 203:813–820
Plaut I (2001) Critical swimming speed: its ecological relevance. Comp Biochem Physiol A 131:41–50
Reidy SP, Kerr SR, Nelson JA (2000) Aerobic and anaerobic swimming performance of individual Atlantic cod. J Exp Biol 203:347–357
Sagnes P, Champagne JY, Morel R (2000) Shifts in drag and swimming potential during grayling ontogenesis: relations with habitat use. J Fish Biol 57:52–68
Sambilay VC (1990) Interrelationships between swimming speed, caudal fin aspect ratio and body length of fishes. Fishbyte 8:16–20
Stobutzki IC (1998) Interspecific variation in sustained swimming ability of late pelagic stage reef fish from two families (Pomacentridae and Chaetodontidae). Coral Reefs 17:111–119
Stobutzki IC, Bellwood DR (1994) An analysis of the sustained swimming abilities of pre- and post-settlement coral reef fishes. J Exp Biol Ecol 175:275–286
Stobutzki IC, Bellwood DR (1997) Sustained swimming abilities of the late pelagic stages of coral reef fishes. Mar Ecol Prog Ser 149:35–41
Wardle CS (1975) Limit of fish swimming speed. Nature 255:725–726
Wardle CS (1977) Effects of size on the swimming speeds of fish. In: Pedley T (ed) Scale effects in animal locomotion. Academic, London
Webb P (1977) Effects of size on performance and energetics of fish. In: Pedley T (ed) Scale effects in animal locomotion. Academic, London
Webb PW (1984) Body form, locomotion and foraging in aquatic vertebrates. Am Zool 24:107–120
Webb P (1994) The biology of fish swimming. In: Maddock L, Bone Q, Rayner J (eds) Mechanics and physiology of animal swimming. Cambridge University Press, Cambridge, pp 45–62
Webb PW, Weihs D (1986) Functional locomotor morphology of early life history stages of fishes. Trans Am Fish Soc 115:115–127
Williams IV, Brett JR (1987) Critical swimming speed of Fraser and Thompson River pink salmon (Oncorhynchus gorbuscha). Can J Fish Aquat Sci 44:348–356
Wilson RW, Egginton S (1994) Assessment of maximum sustainable swimming performance in rainbow trout (Oncorhynchus mykiss). J Exp Biol 192:299–305
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice Hall, New Jersey
Acknowledgements
We thank I.C. Stobutzki, D.R. Bellwood and M. McCormick for use of experimental equipment. Valuable field assistance was provided by S. Street, P. Hansen, H. Parks, K. Hutson, S. Golding, D. Fisher and R. Ferris. We also gratefully acknowledge field logistical support by the Lizard Island Research Station (Australian Museum) as well as the Centre for Marine Resource Studies (The School for Field Studies), South Caicos Island. The Department of Environment and Coastal Resources (South Caicos Island) generously provided access to aquarium facilities. Some experimental specimens were provided by S. Simpson and O. Haine. We also thank D. Wilson for valuable comments on the manuscript and assistance with larval identification of Caribbean species. This work was funded by a Lizard Island Doctoral Fellowship (Australian Museum) (RF), the Australian Coral Reef Society (RF), an ARC Discovery Grant (DP0345876) (JML) and a DST International Science Linkages Programme (ISL-CG03-0043) (JML). Portions of this work were carried out under Australian Museum Animal Care and Ethics Approval 01/01 (JML) and James Cook Ethics Approval A202, 402 (RF).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. W. Sammarco, Chauvin
Rights and permissions
About this article
Cite this article
Fisher, R., Leis, J.M., Clark, D.L. et al. Critical swimming speeds of late-stage coral reef fish larvae: variation within species, among species and between locations. Marine Biology 147, 1201–1212 (2005). https://doi.org/10.1007/s00227-005-0001-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-0001-x