Abstract
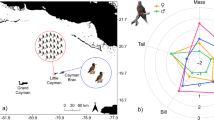
Body size in animals varies with many parameters, amongst them taxonomic affiliation, lifestyle and ambient environment oxygen levels. Size has considerable implication to possibilities for animals; for example, parasites need to be small and top predators large. Body size and resource requirements (shell size) were investigated across the land–sea interface in hermit crabs (Crustacea: Malacostraca: Decapoda) and snails (Mollusca: Gastropoda: Prosobranchia). These are two of the few taxa to occur in the sea, on the shore and on land as residents. Both taxa are also appropriate for such an analysis as they are abundant, speciose, cohabit the same environments and are linked—gastropod shells are a critical resource to hermit crabs. Both the maximum and mean sizes of hermit crab species showed parabolic relationships with shore height, decreasing from the sublittoral and supralittoral to the eulittoral. Average maximum size of gastropods exhibited a similar intertidal minimum although variability was high. It is suggested that this pattern is robust: not only did two distantly related taxa show the same pattern, but neither region nor site contributed significantly to total variability. The mass of resources (gastropod shells) used by hermit crabs, however, showed a converse pattern. The smallest shells (relative to hermit crab body size) were used in the sublittoral and supralittoral. Response to environmental stress and predation pressure are offered as two alternate theories to explain the observed body dwarfism and resource gigantism in the intertidal zone.




Similar content being viewed by others
References
Anderson RP, Handley CO (2002) Dwarfism in insular sloths: biogeography, selection and evolutionary rate. Evolution 56:1045–1058
Atkinson D, Sibley RM (1997) Why are organisms usually bigger in colder environments? Making sense of a life history puzzle. Trends Ecol Evol 12:235–239
Barnes DKA (1997) Ecology of tropical hermit crabs at Quirimba Island, Mozambique: distribution, abundance and activity. Mar Ecol Prog Ser 154:133–142
Barnes DKA (1999) Ecology of tropical hermit crabs at Quirimba Island, Mozambique: shell characteristics and utilisation. Mar Ecol Prog Ser 183:241–251
Barnes DKA, Arnold RJ (2001) Competition, sublethal mortality and diversity on Southern Ocean coastal boulder communities. Polar Biol 24:447–454
Barnes DKA, DeGrave S (2002) Temporospatial constraints in resources available to and used by hermit crabs: tests of models. Funct Ecol 16:714–726
Barnes RSK (1973) The intertidal lamellibranchs of Southampton Water, with particular reference to Cerastoderma edule and C. glaucum. Proc Malacol Soc Lond 40:413–433
Bell JJ, Barnes DKA, Turner J (2002) The importance of macro- and micro-morphological variation in the adaptation of a sublittoral demosponge to current extremes. Mar Biol 140:75–81
Bertness MD (1982) Shell utilization, predation pressure, and thermal stress in Panamanian hermit crabs: an interoceanic comparison. J Exp Mar Biol Ecol 64:159–187
Borjesson DL, Szelistowski WA (1989) Shell selection, utilization and predation in the hermit crab Clibanarius panamensis Stimpson in a tropical mangrove estuary. J Exp Mar Biol Ecol 133:213–228
Burness GP, Diammond J, Flannery T (2001) Dinosaurs, dragons and dwarfs: the evolution of maximal body size. Proc Natl Acad Sci 98:14518–14523
Chapelle G, Peck LS (1999) Polar gigantism dictated by oxygen availability. Nature 399:114–115
Damuth J (1993) Cope’s rule, the island rule and the scaling of mammalian population density. Nature 365:748–750
De Boer WF (1999) Between the tides. Universal Press, Veenedaal, The Netherlands
Foster JB (1964) Evolution of mammals on islands. Nature 202:234–235
Garrity SD (1984) Some adaptations of gastropods to physical stress on a tropical rocky shore. Ecology 65:559–574
Hazlett BA (1981) The behavioural ecology of hermit crabs. Annu Rev Ecol Syst 12:1–22
Helmuth B (1999) Thermal ecology of rocky intertidal mussels: quantifying body temperatures using climatological data. Ecology 80:15–34
Hoffman GE (1999) Ecologically relevant variation in induction and function of heat shock proteins in marine organisms. Am Zool 39:889–900
Hoffman GE, Somero GN (1999) Evidence for protein damage at environmental temperatures: seasonal changes in levels of ubiquitin conjugates and Hsp70 in the intertidal mussel, Mytilus trosulus. J Exp Biol 198:1509–1518
Ishimatsu A, Hishida Y, Takita T, Kanda T, Oikawa S, Takeda T, Huat KK (1998) Mudskippers store air in their burrows. Nature 391:237–238
Jensen K (1970) The interaction between Pagurus bernhardus (L.) and Hydractinia echinata (Fleming). Ophelia 8:135–144
Kellogg CW (1976) Gastropod shells: a potentially limiting resource for hermit crabs. J Exp Mar Biol Ecol 22:101–111
Kreutzer U, Siegmund B, Grieshaber MK (1985) Role of coupled substrates and alternative end products in hypoxia tolerance of marine invertebrates. Mol Physiol 8:371–392
Leite FPP, Turra A, Gandolfi SM (1998) Hermit crabs (Crustacea: Decapoda: Anomura), gastropod shells and environmental structure: their relationship in southeastern Brazil. J Nat Hist 32:1599–1608
Maughan B, Barnes DKA (2000) A ‘minimum stress inflexion’ in relation to environmental and biological influences on the dynamics of subtidal encrusting communities. Hydrobiologia 440:101–109
McGuiness KA (1990) Physical variability, diversity gradients and the ecology of temperate and tropical reefs. Aust J Ecol 15:465–476
Palmer (2002)
Peck LS (1993) The tissues of articulate brachiopods and their value to predators. Philos Trans R Soc Lond B Biol Sci339:17–32
Pörtner HO, Kreutzer U, Siegmund B, Heisler N, Grieshaber MK (1984) Metabolic adaptations of the intertidal worm Sipunculus nudus to functional and environmental hypoxia. Mar Biol 79:237–247
Remane A (1934) Die Brackwasserfauna. Verh Dtsch Zool Ges 36:34–74
Turra A, Leite FPP (2001) Shell utilisation patterns of a tropical rocky intertidal hermit crab assemblage. 1. The case of Grande Beach. J Crustac Biol 21:393–406
Vermeij GJ (1976) Interoceanic differences in vulnerability of shelled prey to crab predation. Nature 260:135–136
Williams JD, McDermott JJ (2004) Hermit crab biocenoses: a worldwide review of the diversity and natural history of hermit crab associates. J Exp Mar Biol Ecol 305:1–128
Zipser E, Vermeij GJ (1978) Crushing behaviour of tropical and temperate crabs. J Exp Mar Biol Ecol 31:155–172
Acknowledgements
I would like to thank Dr P. Convey, Professor L. Peck and two anonymous referees for comments leading to an improved manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.P. Thorpe, Port Erin
Rights and permissions
About this article
Cite this article
Barnes, D.K.A. Body and resource size at the land–sea interface. Marine Biology 146, 625–632 (2005). https://doi.org/10.1007/s00227-004-1451-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-004-1451-2