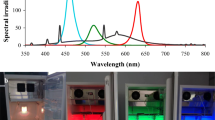
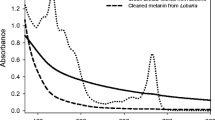
Abstract
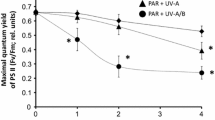
Spores of five Laminariales from Arctic Spitsbergen were exposed in the laboratory to photosynthetically active radiation (PAR; 400–700 nm), PAR+UVA radiation (UVAR; 320–400 nm) and PAR+UVAR+UVB radiation (UVBR; 280–320 nm). Subsequently, germination was monitored over periods of 3, 6 and 9 days. The investigated species were the upper sublittoral Saccorhiza dermatodea, the upper to mid-sublittoral Alaria esculenta and Laminaria digitata, the mid-sublittoral L. saccharina and the lower sublittoral L. solidungula. The germination capacity decreased sharply after 16 h exposure to PAR+UVAR+UVBR in all species. However, S. dermatodea was able to recover from the damaging effects of UVBR. There was also a small increase in percentage germination of A. esculenta 6–9 days after the treatment. No recovery was evident in the other species. After 8 h exposure to PAR+UVA+UVB, L. digitata recovered completely, and L. saccharina and L. solidungula, partially. The only species susceptible to PAR+UVAR was L. solidungula. One prominent cytological feature of UVR-exposed spores was the enlargement of phenolic vesicles (physodes) (particularly seen in S. dermatodea and A. esculenta), which may have a protective function against UVR. Pilot experiments under natural irradiance conditions indicate that the PAR component of solar radiation exerts an additional stress. Overall the data show that zoospores of the species from the upper sublittoral are less sensitive to UVR or have the capacity to recover from UV stress in contrast to species from deeper waters, probably due to their UV protective and repair capabilities.



Similar content being viewed by others
References
Aguilera J, Karsten U, Lippert H, Vögele B, Philipp E, Hanelt D, Wiencke C (1999) Effects of solar radiation on growth, photosynthesis and respiration of marine macroalgae from the Arctic. Mar Ecol Prog Ser 191:109–119
Aguilera J, Dummermuth A, Karsten U, Schriek R, Wiencke C (2002) Enzymatic defenses against photooxidative stress induced by ultraviolet radiation in Arctic marine macroalgae. Polar Biol 25:432–441
Altamirano M, Flores-Moya F, Figueroa FL (2000) Long-term effects of natural sunlight under various ultraviolet radiation conditions on growth and photosynthesis of intertidal Ulva rigida (Chlorophyceae) cultivated in situ. Bot Mar 43:19–126
Bischof K, Hanelt D., Wiencke C (1998a) UV-radiation can affect depth-zonation of Antarctic macroalgae. Mar Biol 131:597–605
Bischof K, Hanelt D, Tüg H, Karsten U, Brouwer PEM, Wiencke C (1998b) Acclimation of brown algal photosynthesis to ultraviolet radiation in Arctic coastal waters (Spitsbergen, Norway). Polar Biol 20:388–395
Bischof K, Hanelt D., Wiencke C (1999) Acclimation of maximal quantum yield of photosynthesis in the brown alga Alaria esculenta under high light and UV radiation. Plant Biol 1:435–444
Bischof K, Hanelt D, Wiencke C. (2000) UV-effects on photosynthesis and related enzyme reactions of marine macroalgae. Planta 211:555–562
Campbell D, Eriksson MJ, Öquist G, Gustafsson P, Clarke AK (1998) The cyanobacterium Synechococcus resists UV-B by exchanging photosystem II reaction-center D1 proteins. Proc Natl Acad Sci 95:364–369
Clayton MN, Wiencke C, Schoenwaelder MEA (2004) UV-absorbing compounds in spores of Arctic Laminariales. Eur J Phycol (in press)
Cockell CS, Knowland J (1999) Ultraviolet radiation screening compounds. Biol Rev 74:311–345
Coelho SM, Rijstenbil JW, Brown MT (2000) Impacts of anthropogenic stresses on the early development stages of seaweeds. J Aquat Ecosyst Stress Recovery 7:317–333
Collen J, Pedersen M (1996) Production, scavenging and toxicity of hydrogen peroxide in the green seaweed Ulva rigida. Eur J Phycol 31:265–271
Dahlback A (2002) Recent changes in surface ultraviolet solar radiation and stratospheric ozone at a high Arctic site. In: Hessen D (ed) UV radiation and Arctic ecosystems. Springer, Berlin Heidelberg New York, pp 3–22
Dring MJ, Wagner A, Boeskov J, Lüning K (1996a) Sensitivity of intertidal and subtidal red algae to UVA and UVB radiation, as monitored by chlorophyll fluorescence measurements: influence of collection depth and season, and length of irradiation. Eur J Phycol 31:293–302
Dring MJ, Makarov V, Schoschina E, Lorenz M, Lüning K (1996b) Influence of ultraviolet-radiation on chlorophyll fluorescence and growth in different life-history stages of three species of Laminaria (Phaeophyta). Mar Biol 126:183–191
Dummermuth AL, Karsten U, Fisch KM, König, GM, Wiencke C. (2003) Responses of marine macroalgae to hydrogen-peroxide-stress. J Exp Mar Biol Ecol 289:103–121
Flores-Moya A, Posudin YI, Fernández JA, Figueroa FL, Kawai H (2002) Photomovement of the swarmers of the brown algae Scytosiphon lomentaria and Petalonia fascia: effect of photon irradiance, spectral composition and UV dose. J Photochem Photobiol B 66:134–140
Foyer CH, Lelandais M, Kunert KJ (1994) Photooxidative stress in plants. Physiol Plant 92:696–717
Franklin LA, Forster RM (1997) The changing irradiance environment: consequences for marine macrophyte physiology, productivity and ecology. Eur J Phycol 32:207–232
Gathen P von der, Rex M, Harris NRP, Lucic D, Knudsen BM, Braathen GO, Backer H De, Fabian R, Fast H, Gil M, Kyrö E, Mikkelsen I St, Rummukainen M, Staehelin J, Varotsos C (1995) Observational evidence for chemical ozone depletion over the Arctic in winter 1991–92. Nature 375:131–134
Groß C, Tüg H, Schrems O (2001) Three years spectral resolved UV-measurements at Koldewey-Station (1997–1999). Mem Natl Inst Polar Res Spec Issue 54:113–123
Han T, Han Y-S, Kim K-Y, Kim J-H, Shin H-W, Kain JM, Callow JA, Callow ME (2003) Influences of light and UV-B on growth and sporulation of the green alga Ulva pertusa Kjellman. J Exp Mar Biol Ecol 4125:1–17
Hanelt D, Wiencke C, Nultsch W (1997) Influence of UV radiation on photosynthesis of Arctic macroalgae in the field. J Photochem Photobiol B Biol 38:40–47
Hanelt D, Tüg GH, Bischof K, Groß C, Lippert H, Sawall T, Wiencke C (2001) Light regime in an Arctic fjord: a study related to stratospheric ozone depletion as a basis for determination of UV effects on algal growth. Mar Biol 138:649–658
Hanken T, Tüg, H (2002) Development of a multichannel UV-spectroradiometer for field measurements. Environ Sci Pollut Res Spec Issue 4:35–39
Hop H, Pearson T, Hegseth EN, Kovacs KM, Wiencke C, Kwasniewski S, Eiane K, Mehlum F, Gulliksen B, Wlodarska-Kowalczuk M, Lydersen C, Weslawski JM, Cochrane S, Gabrielsen GW, Leakey R, Lønne OJ, Zajaczkowski M, Falk-Petersen S, Kendall M, Wängberg SÅ, Bischof K, Voronkov AY, Kovaltchouk NA, Wiktor J, Poltermann M, di Prisco G, Papucci C, Gerland, S (2002) The ecosystem of Kongsfjorden, Svalbard. Polar Res 21:167–208
Huovinen PS, Oikari AOJ, Soimasuo MR, Cherr GN (2000) Impact of UV radiation on the early development of the giant kelp (Macrocystis pyrifera) gametophytes. Photochem Photobiol 72:308–313
Karsten, U, Sawall T, Hanelt D, Bischof K, Figueroa FL, Flores-Moya A, Wiencke C (1998) An inventory of UV-absorbing mycosporine-like amino acids in macroalgae from polar to warm-temperate regions. Bot Mar 41:443–453
Karsten U, Bischof K, Hanelt D, Tüg H, Wiencke C (1999) The effect of UV radiation on photosynthesis and UV-absorbing substances in the endemic Arctic macroalga Develaraea ramentacea (Rhodophyta). Physiol Plant 105:58–66
Karsten U, Bischof K, Wiencke C (2001) Photosynthetic performance of Arctic macroalgae after transplantation from deep to shallow waters followed by exposure to natural solar radiation. Oecologia 127:11–20
Lüning K (1980) Critical levels of light and temperature regulating the gametogenesis of three Laminaria species (Phaeophyceae). J Phycol 16:1–15
Makarov MV, Voskoboinikov GM (2001) The influence of ultraviolet-B radiation on spore release and growth of the kelp Laminaria saccharina. Bot Mar 44:89–94
Máté Z, Sass L, Szekeres M, Vass I, Nagy F (1998) UV-B induced differential transcription of psbA genes encoding the D1 protein of photosystem II in the cyanobacterium Synechocystis 6803. J Biol Chem 273:17439–17444
Pakker H, Beekman CAC, Breeman AM (2000a) Efficient photoreactivation of UVBR-induced DNA damage in the sublittoral macroalga Rhodymenia pseudopalmata (Rhodophyta). Eur J Phycol 35:109–114
Pakker H, Martins RST, Boelen P, Buma AGJ, Nikaido O, Breeman AM (2000b) Effects of temperature on the photoreactivation of ultraviolet-B-induced DNA damage in Palmaria palmata (Rhodophyta). J Phycol 36:334–341
Pavia H, Brock E (2000) Extrinsic factors influencing phlorotannin production in the brown alga Ascophyllum nodosum. Mar Ecol Prog Ser 193:285–294
Pavia H, Cervin G, Lindgren A, Åberg P (1997) Effects of UV-B radiation and simulated herbivory on phlorotannins in the brown alga Ascophyllum nodosum. Mar Ecol Prog Ser 157:139–146
Peckol P, Krane JM, Yates JL (1996) Interactive effects of inducible defense and resource availability on phlorotannins in the North Atlantic brown alga Fucus vesiculosus. Mar Ecol Prog Ser 138:209–217
Poll WH van de, Bischof, K, Buma AGJ, Breeman AM (2002a) Habitat related variation in UV tolerance of tropical marine red macrophytes is not temperature dependent. Physiol Plant 118:74–83
Poll WH van de, Eggert A, Buma AGJ, Breeman AM (2002b) Effects of UV-B induced DNA damage and photoinhibition on growth of temperate marine red macrophytes: habitat-related differences in UV-B tolerance. J Phycol 37:30–37
Poll WH van de, Eggert A, Buma AGJ, Breeman AM (2002c) Temperature dependence of UV radiation effects in Arctic and temperate isolates of three red macrophytes. Eur J Phycol 37:59–68
Poll WH van de, Hanelt D, Hoyer K, Buma AGJ, Breeman AM (2002d) Ultraviolet-B induces cyclobutane-pyrimidine dimer formation and repair in Arctic marine macrophytes. Photochem Photobiol 76:493–501
Ragan MA, Glombitza KW (1986) Phlorotannins, brown algal polyphenols. In: Round FE, Chapman DJ (eds) Progress in phychological research 4. Biopress, Bristol, pp 129-241
Rex M, Salawitch RJ, Harris NRP, Gathen P von der, Braathen GO, Schulz A, Deckelmann H, Chipperfield M, Sinnhuber BM, Reimer E, Alfier R, Bevilacqua R,Hoppel K, Fromm M, Lumpe J, Küllmann H,Kleinböhl A, Bremer H, König M, Künzi K, Toohey D, Vömel H, Richard E, Aikin K, Jost H, Greenblatt JB, Loewenstein M, Podolske JR, Webster CR, Flesch GJ, Scott DC, Herman R, Margitan L, Elkins JW, Ray EA, Moore FL, Hurst DF, Romashkin P, Toon GC, Sen BJJ, Wennberg P, Neuber R, Allart M, Bojkov RB, Claude H, Davies J, Davies W, Backer H, de Dier H, Dorokhov V, Fast, H, Kondo Y, Kyrö E, Litynska Z, Mikkelsen IS, Molyneux MJ, Moran E, Murphy G, Nagai T, Nakane H, Parrondo C, Ravegnani F, Skrivankova P, Viatte P, Yushkov V (2002) Chemical loss of Arctic ozone in winter. 1999/2000. J Geophys Res 107/D20:8276
Roy CR, Gies HP, Elliot G (1990) Ozone depletion. Nature 347:235–6
Schoenwaelder ME (2002a) The occurrence and cellular significance of physodes in brown algae. Phycologia 41:125–139
Schoenwaelder ME (2002b) Physode distribution and the effect of ‘thallus sunburn’ in Homosira banksii (Fucales, Phaeophyceae). Bot Mar 45:262–266
Solomon S (1999) Statospheric ozone depletion: a review of concepts and history. Rev Geophys 37:275–316
Staehelin J, Harris NRP, Appenzeller C, Eberhard J (2001) Ozone trends: a review. Rev Geophys 39:231–290
Starr RC, Zeikus JA (1987) UTEX—The cultural collection of algae at the University of Texas at Austin. J Phycol [Suppl] 23:1–47
Svendsen H, Beszczynska-Møller A, Hagen JO, Lefauconnier B, Tverberg V, Gerland S, Ørbaek JB, Bischof K, Papucci C, Zajaczkowski M, Azzolini R, Bruland O, Wiencke C, Winther J, Dallmann W (2002) The physical environment of Kongsfjorden–Krossfjorden, an Arctic fjord system in Svalbard. Polar Res 21:133–166
Wiencke C, Gómez I, Pakker H, Flores-Moya A, Altamirano M, Hanelt D Bischof, K, Figueroa FL (2000) Impact of UV radiation on viability, photosynthetic characteristics and DNA of brown algal zoospores: implications for depth zonation. Mar Ecol Prog Ser 197:217–229
Acknowledgements
This work was performed at the Ny Ålesund International Research and Monitoring Facility on Spitsbergen (Svalbard). The authors are grateful to M. Schwanitz, C. Daniel, M. Assmann, H. Wessels and H. Schmidt for providing samples by SCUBA diving, as well as to the staff at Koldewey Station, especially H. Pötschick. The experiments comply with the current laws of Germany and Norway.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Kinne, Oldendorf/Luhe
Rights and permissions
About this article
Cite this article
Wiencke, C., Clayton, M.N. & Schoenwaelder, M. Sensitivity and acclimation to UV radiation of zoospores from five species of Laminariales from the Arctic. Marine Biology 145, 31–39 (2004). https://doi.org/10.1007/s00227-004-1307-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-004-1307-9