Abstract
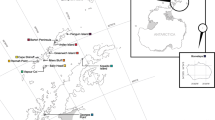
We measured within- and among-population genetic variation in the green sea urchin (Strongylocentrotus droebachiensis) at 11 sites in the north Atlantic and northeast Pacific by using four-locus microsatellite genotypes. We found no differentiation among populations from Atlantic Canada, but strong differentiation across the north Atlantic and between the Atlantic and Pacific samples. High inbreeding coefficients at three loci are consistent with high variance in reproductive success. One population that was recently decimated by disease was strongly differentiated from some others, but there was little differentiation otherwise among populations in Atlantic Canada. On a larger scale, populations in Atlantic Canada were more similar to a population from the north Pacific than to populations in the northwest Atlantic. Differentiation among populations at this large spatial scale is consistent with biogeographical hypotheses of: (1) Pleistocene population reduction and isolation in the northeast Atlantic, but (2) extinction in the northwest Atlantic followed by extensive recolonization from the Pacific. In contrast to other recent studies of trans-Atlantic organisms, we found no evidence of extensive gene flow across the north Atlantic.


Similar content being viewed by others
References
Addison JA, Hart MW (2002) Characterization of microsatellite loci in sea urchins (Strongylocentrotus spp.). Mol Ecol Notes 2:493–494
Balch T, Scheibling RE (2000) Temporal and spatial variability in settlement and recruitment of echinoderms in kelp beds and barrens in Nova Scotia. Mar Ecol Prog Ser 205:139–154
Balch T, Scheibling RE, Harris LG, Chester CM, Robinson SMC (1998) Variation in settlement of Strongylocentrotus droebachiensis in the northwest Atlantic: effects of spatial scale and sampling method. In: Mooi R, Telford M (eds) Echinoderms. Balkema, Rotterdam, pp 555–560
Beacham TD, Brattey J, Miller KM, Le KD, Withler RE (2002) Multiple stock structure of Atlantic cod (Gadus morhua) off Newfoundland and Labrador determined from genetic variation. ICES J Mar Sci 59:650–655
Bentzen P, Taggart CT, Ruzzante DE, Cook D (1996) Microsatellite polymorphism and the population structure of Atlantic cod (Gadus morhua) in the northwest Atlantic. Can J Fish Aquat Sci 53:2706–2721
Benzie JAH, Stoddart JA (1992) Genetic structure of outbreaking and non-outbreaking crown-of-thorns starfish (Acanthaster planci) populations on the Great Barrier Reef. Mar Biol 112:119–130
Bohonak AJ (1999) Dispersal, gene flow, and population structure. Q Rev Biol 74:21–45
Burton RS (1983) Protein polymorphisms and genetic differentiation of marine invertebrate populations. Mar Biol Lett 4:193–206
Chapman ARO (1981) Stability of sea urchin dominated barren grounds following destructive grazing of kelp in St. Margaret's Bay, eastern Canada. Mar Biol 62:307–311
Debenham P, Brzezinski M, Foltz K, Gaines S (2000) Genetic structure of populations of the red sea urchin, Strongylocentrotus franciscanus. J Exp Mar Biol Ecol 253:49–62
Dufresne F, Bourget E, Bernatchez L (2002) Differential patterns of spatial divergence in microsatellite and allozyme alleles: further evidence for locus-specific selection in the acorn barnacle, Semibalanus balanoides? Mol Ecol 11:113–123
Durham JW, MacNeil FS (1967) Cenozoic migrations of marine invertebrates through the Bering Strait region. In: Hopkins DM (ed) The Bering land bridge. Stanford University Press, Stanford, Calif., pp 326–349
Edmands S, Moberg PE, Burton RS (1996) Allozyme and mitochondrial DNA evidence of population subdivision in the purple sea urchin Strongylocentrotus purpuratus. Mar Biol 126:443–450
Flowers JM, Schroeter SC, Burton RS (2002) The recruitment sweepstakes has many winners: genetic evidence from the sea urchin Strongylocentrotus purpuratus. Evolution 56:1445–1453
Gladenkov YB (1979) Cenozoic molluscan assembledges in northern regions of the Atlantic and Pacific Oceans. Int Geol Rev 21:880–890
Goudet J (2001) FSTAT, a program to estimate and test gene diversities and fixation indices (version 2.9.3). Available from http://www.unil.ch/izea/softwares/fstat.html
Grosberg RK (1996) Simple extractions and RAPD-PCR protocols. In: Ferraris JD, Palumbi SR (eds) Molecular zoology: advances, strategies, and protocols. Wiley-Liss, New York, pp 470–473
Harris LG, Rice B, Nestler EC (1994) Settlement, early survival and growth in a southern Gulf of Maine population of Strongylocentrotus droebachiensis (Müller). In: David B, Guille A, Feral J-P, Roux M (eds) Echinoderms through time. Balkema, Rotterdam, pp 701–706
Hart MW, Scheibling RE (1988) Heat waves, baby booms, and the destruction of kelp beds by sea urchins. Mar Biol 99:167–176
Hatcher BG, Hatcher AI (1997) Research directions and management options for sea urchin culture in Nova Scotia. Bull Aquacult Assoc Can 97:62–65
Hedgecock D (1994) Does variance in reproductive success limit effective population sizes of marine organisms? In: Beaumont AR (ed) Genetics and evolution of aquatic organisms. Chapman and Hall, London, pp 122–134
Hellberg ME, Burton RS, Neigel JE, Palumbi SR (2002) Genetic assessment of connectivity among marine populations. Bull Mar Sci 70[Suppl]:273–290
Herbinger CM, Vercaemer BM, Gjetvaj B, O'Dor RK (1998) Absence of genetic differentiation among geographically close sea scallop (Placopecten magellanicus) beds with cDNA and microsatellite markers. J Shellfish Res 17:117–122
Johnson CR, Mann KH (1988) Diversity, patterns of adaptation, and stability of Nova Scotia kelp beds. Ecol Monogr 58:129–154
Johnson MS, Black R (1982) Chaotic genetic patchiness in an intertidal limpet, Siphonaria sp. Mar Biol 70:157–164
Johnson MS, Black R (1984a) Pattern beneath the chaos: the effect of recruitment on genetic patchiness in an intertidal limpet. Evolution 38:1371–1383
Johnson MS, Black R (1984b) The Wahlund effect and the geographical scale of variation in the intertidal limpet Siphonaria sp. Mar Biol 79:295–302
Knutsen H, Jorde PE, Andre C, Stenseth NC (2003) Fine-scaled geographical population structuring in a highly mobile marine species: the Atlantic cod. Mol Ecol 12:385–394
Lewis KM, Feder JL, Lamberti GA (2000) Population genetics of the zebra mussel, Dreissena polymorpha (Pallas): local allozyme differentiation within midwestern lakes and streams. Can J Fish Aquat Sci 57:637–643
Lewis PO, Zaykin D (2001) Genetic Data Analysis: computer program for the analysis of allelic data, version 1.0 (d16c). Free program distributed by the authors via internet from http://lewis.eeb.uconn.edu/lewishome/software.html
Marcus NH (1977) Genetic variation within and between geographically separated populations of the sea urchin, Arbacia punctulata. Biol Bull (Woods Hole) 153:560–576
McPherson AA, Stephenson RL, O'Reilly PT, Jones MW, Taggart CT (2001) Genetic diversity of coastal northwest Atlantic herring populations: implications for management. J Fish Biol 59:356–370
Meidel SK, Scheibling RE (1998) Size and age structure of the sea urchin Strongylocentrotus droebachiensis in different habitats. In: Mooi R, Telford M (eds) Echinoderms. Balkema, Rotterdam, pp 737–742
Miller RJ (1985) Succession in sea urchin abundance in Nova Scotia, Canada. Mar Biol 84:275–286
Mladenov PV, Allibone RM, Wallis GP (1997) Genetic differentiation in the New Zealand sea urchin Evechinus chloroticus (Echinodermata: Echinoidea). NZ J Mar Freshw Res 31:261–269
Moberg PE, Burton RS (2000) Genetic heterogeneity among adult and recruit red sea urchins, Strongylocentrotus franciscanus. Mar Biol 136:773–784
Palumbi SR (1995) Using genetics as an indirect estimator of larval dispersal. In: McEdward LR (ed) Ecology of marine invertebrate larvae. CRC Press, Boca Raton, Fla., pp 369–387
Palumbi SR, Kessing BD (1991) Population biology of the trans-Arctic exchange: mtDNA sequence similarity between Pacific and Atlantic sea urchins. Evolution 45:1790–1805
Palumbi SR, Wilson AC (1990) Mitochondrial DNA diversity in the sea urchins Strongylocentrotus purpuratus and S. droebachiensis. Evolution 44:403–415
Petrie B (1987) Undulations of the Nova Scotia current. Atmos-Ocean 25:1–9
Pogson GH, Taggart CT, Mesa KA, Boutilier RG (2001) Isolation by distance in the Atlantic cod, Gadus morhua, at large and small spatial scales. Evolution 55:131–146
Pringle JD, Sharp GJ, Caddy JF (1982) Interactions in kelp bed ecosystems in the northwest Atlantic: review of a workshop. In: Mercer MC (ed) Multispecies approaches to fisheries management advice. Can Spec Publ Fish Aquat Sci 59:108–115
Raymond M, Rousset F (1995) GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Rice WR (1989) Analysing tables of statistical tests. Evolution 43:223–225
Scheibling RE, Hennigar AW (1997) Recurrent outbreaks of disease in sea urchins Strongylocentrotus droebachiensis in Nova Scotia: evidence for a link with large-scale meteorologic and oceanographic events. Mar Ecol Prog Ser 152:155–165
Scheibling RE, Raymond BG (1990) Community dynamics on a subtidal cobble bed following mass mortalities of sea urchins. Mar Ecol Prog Ser 63:127–145
Strathmann RR (1978) Length of pelagic period in echinoderms with feeding larvae from the northwest Pacific. J Exp Mar Biol Ecol 34:23–27
Swofford DL (2002) PAUP*: phylogenetic analysis using parsimony (* and other methods), version 4. Sinauer, Sunderland, Mass.
Vermeij GJ (1979) Anatomy of an invasion: the trans-Arctic interchange. Paleobiology 17:281–307
Wares JP, Cunningham CW (2001) Phylogeography and historical ecology of the north Atlantic intertidal. Evolution 55:2455–2469
Watts RJ, Johnson MS, Black R (1990) Effects of recruitment on genetic patchiness in the urchin Echinometra mathaei in Western Australia. Mar Biol 105:145–151
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Zouros E, Foltz DW (1984) Possible explanations of heterozygote deficiency in bivalve molluscs. Malacologia 25:581–591
Acknowledgements
We thank T. Balch, C. Begin, N. Hagen, J. Svavarsson, and B. Hooper for providing samples; F. Harper, S. Watts, and J. Lindley for assistance collecting samples using SCUBA; D. Duggins and the Friday Harbor Laboratories for dredge collections; D. Cook for valuable technical support; J. Lake for laboratory help; and two anonymous reviewers for comments on the manuscript. This work was supported by the Natural Sciences and Engineering Research Council of Canada, Canada Foundation for Innovation, the Nova Scotia Department of Economic Development, and the Timiskaming First Nation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R.J. Thompson, St. John's
Rights and permissions
About this article
Cite this article
Addison, J.A., Hart, M.W. Analysis of population genetic structure of the green sea urchin (Strongylocentrotus droebachiensis) using microsatellites. Marine Biology 144, 243–251 (2004). https://doi.org/10.1007/s00227-003-1193-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-003-1193-6