Abstract
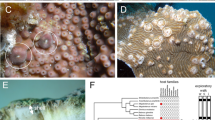
This article describes ecological and biological differences between two morphs of the Red Sea fire coral Millepora dichotoma. The species is divided into two main morphs: branching and encrusting, which were found to differ both in color and morphology. Each morph has two or more sub-morphs. A total of 372 M. dichotoma colonies were examined in a census at two study sites in the Gulf of Elat. Colony size and abundance of the two morphs were found to differ significantly between sites. Experimental examination of each morph's morphological plasticity revealed different growth rates and difference in growth plasticity between the branching and the encrusting morph. Most of the fragments from the branching colonies (94%) attached to experimentally placed Plexiglas substrate, compared with much less attachment by the encrusting fragments (11%). The growth form of the branching morph on the Plexiglas switched to encrusting, spreading over and covering the substrate. When the new encrusting colony reached the edge of this substrate, it started to produce tips, and returned to growth in the classic branching form. The encrusting morph did not change its growth form. Following attachment of the original fragments of the branching morph to the substrate, 8.1% of them produced new tips. When the original branches were removed, after converting to encrusting growth form, 19% of the fragments produced new tips. The capsule size of nematocysts of the two morphs was also significantly different (t-test, P<0.05). Molecular data (ITS region) clearly demonstrate that these two M. dichotoma morphs differ considerably. Molecular evidence (srRNA) from the symbiotic zooxanthellae also shows a different pattern of clades in the hosts. The ecological, biological and molecular data thus attest to the two morphs being distinguishable. Contrary to previous reports, we consequently suggest that the two morphs of M. dichotoma found in the Gulf of Elat are actually two distinct species.







Similar content being viewed by others
References
Amaral FD (1994) Morphological variation in the reef coral Montastrea cavernosa in Brazil. Coral Reefs 13:113–117
Amaral FD, Silva RS, Mauricio-da-Silva L, Sole-Cava AM (1997) Molecular systematics of Millepora alcicornis Linnaeus, 1758 and M. braziliensis Verrill, 1868 (Hydrozoa: Milleporidae) from Brazil. Proc 8th Int Coral Reef Symp vol II, pp 1577–1580
Arsenault DJ, Marchinko KB, Palmer R (2001) Precise tuning of barnacle leg length to coastal wave action. Proc R Soc Lond B 268:2149–2154
Barata C, Baird DJ, Soares AMVM (2001) Phenotypic plasticity in Daphnia magna Straus: variable maturation instar as an adaptive response to predation pressure. Oecologia 129:220–227
Beltran-Torres AU, Carricart-Ganivet JP (1993) Skeletal morphologic variation in Montastrea cavernosa (Cnidaria, Scleractinia) at Isla Verde coral reef, Veracruz, Mexico. Rev Biol Trop 41(3A):559–562
Boschma H (1948) The species problem in Millepora. Zool Verh Leiden 1:1-115
Boschma H (1962) On milleporine corals from Brazil. K Ned Akad Wet Amst Proc 65C:302–312
Bosscher H, Meesters EH (1992) Depth related changes in the growth rate of Montastrea annularis. Proc 7th Int Coral Reef Symp 1:507–512
Bruno JF, Edmunds PJ (1997) Clonal variation for phenotypic plasticity in the coral Madracis mirabilis. Ecology 78:2177–2190
Chen CA, Willis BL, Miller DJ (1996) Systematic relationships between tropical corallimorpharians (Cnidaria: Anthozoa: Corallimorpharia): utility of the 5.8S and internal transcribed spacer (ITS) refions of the rRNA transcription unit. Bull Mar Sci 59:196–208
Chen CA, Chen CP, Fan TY, Yu JK, Hsieh HL (2002) Nucleotide sequences of ribosomal internal transcribed spacers and their utility in distinguishing closely related Perinereis polychaets (Annelida; Polychaeta; Nereididae).
Edmunds PJ (1999) The role of colony morphology and substratum inclination in the success of Millepora alcicornis on shallow coral reefs. Coral Reefs 18:133–140
Graus RR, MacIntyre IG (1982) Variation in the growth forms of the reef coral Montastrea annularis (Ellis and Solander): a quantitative evaluation of growth response to light distribution using computer simulation. Smithson Contrib Mar Sci 12:441–464
Lesser MP, Weis VM, Patterson MR, Jokiel PL (1994) Effects of morphology and water motion on carbon delivery and productivity in the reef coral, Pocillopora-damicornis (Linnaeus) - diffusion-barriers, inorganic carbon limitation, and biochemical plasticity. J Exp Mar Biol Ecol 178:153–179
Lewis JB (1989) The ecology of Millepora. Coral Reefs 8:99–107
Manchenko GP, Moschenko AV, Odintsov VS (1993) Biochemical genetics and systematics of Millepora (Coelenterata: Hydrozoa) from the shore of south Vietnam. Biochem Syst Ecol 21:729–735
Mariscal RN (1974) Nematocysts. In: Muscatine L, Lenhoff HM (eds) Coelenterate biology. Academic, New York, pp 129–178
Medina M, Weil E, Szmant AM (1999) Examination of the Montastraea annularis species complex (Cnidaria: Scleractinia) using ITS and COI sequences. Mar Biotechnol 1:89–97
Mokady O, Brickner I (2001) Host-associated speciation in a coral inhabiting barnacle. Mol Biol Evol 18:975–981
Mokady O, Loya Y, Achituv Y, Geffen E, Graur D, Rozenblatt S, Brickner I (1999) Speciation versus phenotypic plasticity in coral inhabiting barnacles-Darwins observations in an ecological context. J Mol Evol 49:367–375
Muko S, Kawasaki K, Sakai K, Takasu F, Shigesada N (2000) Morphological plasticity in the coral Porites sillimaniani and its adaptive significance. Bull Mar Sci 66:225–239
Odorico DM, Miller DJ (1997a) Variation in the ribosomal internal transcribed spacers and 5.8S rDNA among five species of Acropora (Cnidaria; Scleractinia): patterns of variation consistent with reticulate evolution. Mol Biol Evol 14:465–473
Odorico DM, Miller DJ (1997b) Internal and external relationships of the Cnidaria: implications of primary and predicted secondary structure of the 5'-end of the 23S-like rDNA. Proc R Soc Lond B Biol Sci 264:77–82
Olsen GJ, Matsuda H, Hagstrom R, Overbeek R (1994) FastDNAml—a tool for construction of phylogenetic trees of DNA-sequences using maximum-likelihood. Comput Appl Biosci 10:41–48
Rowan R, Powers DA (1991) Molecular genetic identification of symbiotic dinoflagellates (zooxanthellae). Mar Ecol Prog Ser 71:65–73
Rowan R, Knowlton N, Baker A, Jara J (1997) Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature 388:265–269
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. A laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Schwaninger HR (1999) Population structure of the widely dispersing marine bryozoan Membranipora membranacea (Cheilostomata): implications for population history, biogeography, and taxonomy. Mar Biol 135:411–423
Smith LD, Palmer AR (1994) Effects of manipulated diet on size and performance of Brachyuran crab claws. Science 264:710–712
Stearn CW, Riding R (1973) Forms of the hydrozoan Millepora on a recent coral reef. Lethaia 6:187–200
Takabayashi M, Carter DA, Loh WKW, Hoegh-Guldberg O (1998) A coral specific primer for PCR amplification of the internal transcribed spacer region in ribosomal DNA. Mol Ecol 7:925–931
Todd PA, Sanderson PG, Chou LM (2001) Morphological variation in the polyps of the scleractinian coral Favia speciosa (Dana) around Singapore. Hydrobiologia 444:227–235
Vago R, Shai Y, Ben-Zion M, Dubinsky Z, Achituv Y (1994) Computerized tomography and image analysis, a tool for examining the skeletal characteristics of reef building organisms. Limnol Oceanogr 39:448–452
Vago R, Achituv Y, Vaky L, Dubinsky Z, Kizner Z (1998) Colony architecture of Millepora dichotoma Forskal. J Exp Mar Biol Ecol 224:225–235
Veron JEN, Odorico DM, Chen CA, Miller DJ (1996) Reassessing evolutionary relationships of scleractinian corals. Coral Reefs 15:1–9
Weerdt WH de (1981) Transplantation experiments with Caribbean Millepora species (Hydrozoa, Coelenterata), including some ecological observations on growth forms. Bijdr Dierkd 51:1–19
Weerdt WH de (1984) Taxonomic characters in Caribbean Millepora species (Hydrozoa, Coelenterata) including some ecological observations on growth forms. Bijdr Dierkd 54:243–362
West JM (1997) Plasticity in the sclerites of a gorgonian coral: tests of water motion, light level, and damage cues. Biol Bull 192:279–289
Acknowledgements
We thank E. Geffen for assistance with the statistics, construction of the phylogentic trees and his important comments on the manuscript. We are grateful to I. Lerer for his invaluable technical assistance. A. Daya assisted with photography. We thank L. Fishelson for his help with the study of the nematocyts. S. Sheffer assistance with DNA extraction is acknowledged. Parts of this research were supported by the Israeli Diving Federation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Kinne, Oldendorf/Luhe
Rights and permissions
About this article
Cite this article
Meroz-Fine, E., Brickner, I., Loya, Y. et al. The hydrozoan coral Millepora dichotoma: speciation or phenotypic plasticity?. Marine Biology 143, 1175–1183 (2003). https://doi.org/10.1007/s00227-003-1135-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-003-1135-3