Abstract
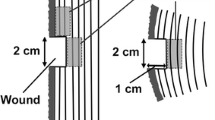
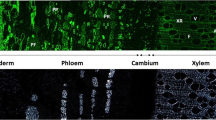
The kinetics of xylem formation in four-year-old plants of Swietenia mahagoni (L.) Jacq. and Khaya ivorensis A. Chev. were studied by means of high resolution laser measurements (accuracy: ±2 μm) in a spatial resolution of 18.7 to 94.1 μm and a temporal resolution of 1 to 60 s. The radial enlargement of the xylem cells was completed within 0.36 to 8.85 hours in Swietenia and within 0.52 to 12.03 hours in Khaya, while secondary wall formation and lignification lasted days to weeks. Cell enlargement of vessels and ray parenchyma was significantly faster than radial enlargement of fibres and axial parenchyma. The processes of formation of the secondary cell wall and the lignification were slower in fibres than in vessels and parenchyma cells. In Swietenia new secondary cell formation was induced in distinct growth periods almost simultaneously in the whole shoot, while in Khaya new cell formation was restricted to parts of the shoot. Growth stresses in the shoot were studied in terms of released strain. Higher growth stresses were observed in the shoot of Khaya compared to Swietenia. The results indicate that the higher growth stresses in the xylem of Khaya compared to Swietenia originate in the different kinetics of cell development of different cell types in combination with the heterogenous sequence of cell formation within the shoot.








Similar content being viewed by others
References
Arévalo R, Hernández RE (2003) Influence of moisture sorption on the tangential compression strength of mahogany wood (Swietenia macrophylla King). Wood Sci Technol 37:419–425
Bailey IW (1919) Phenomena of cell division in the cambium of arborescent gymnosperms and their cytological significance. Proc Nat Acad Sci 5:283–285
Barnett JR (1992) Reactivation of the cambium in Aesculus hippocastanum L.: A transmission electron microscope study. Ann Bot 70:169–177
Boyd JD (1950a) Tree growth stresses-the development of shakes and other visual failures. Austr J Sci Res 1:296–312
Boyd JD (1950b) Tree growth stresses-the origin of growth stresses. Austr J Sci Res 3:293–309
Burgert I, Bernasconi A, Niklas KJ, Eckstein D (2001) The influence of rays on the transverse elastic anisotropy in green wood of deciduous trees. Holzforschung 55:49–454
Carl KY, McAinsh MR (2003) Encoding specificity in plant calcium signalling: Hot-spotting the ups and downs and waves. Ann Bot 92:477–485
Catesson AM (1962) Modalité de l’activité proliferatrice du cambium d’acer pseudoplatanus au cours du cycle annuel. Acad Sci 255:3462–3464
Catesson AM (1989) Specific characters of vessel primary walls during early stages of wood differentiation. Biol of Cell 67:221–226
Catesson AM (1994) Cambial ultrastructure and biochemistry: Changes in relation to vascular tissue differentiation and the seasonal cycle. Int J Plant Sci 155:251–261
Coster C (1927) Zur Anatomie und Physiologie der Zuwachszonen und Jahresringbildung in den Tropen 1. Ann Jard Buitenzorg 37:49-161
Dill-Langer G, Lütze S, Aicher S (2002) Microfracture in wood monitored by confocal laser scanning microscopy. Wood Sci Technol 36:487–499
Dodd RS, Fox P (1990) Kinetics of tracheid differentiation in Douglas-fir. Ann Bot 65:649–657
Dupuy B, Koua M (1993) Les plantations d’acajou d’Afrique. Bois Forêts Trop 236:25–41
Dünisch O, Bauch J (1994) Influence of soil substrate and drought on wood formation of spruce (Picea abies [L.] Karst.) under controlled conditions. Holzforschung 48:447–457
Dünisch O, Bauch J, Gasparotto L (2001) Formation of increment zones and intraannual growth dynamics in the xylem of Swietenia macrophylla King, Carapa guianensis Aubl., and Cedrela odorata L. (Meliaceae). IAWA J 23:101–119
Dünisch O, Schulte M, Kruse K (2003) Cambial growth of Swietenia macrophylla King studied under controlled conditions by high resolution laser measurements. Holzforschung 57:196–206
Esau K (1970) Plant anatomy. Academic Press, Orlando, pp 231–258
Farrar JJ, Evert RF (1997a) Seasonal changes in the ultrastructure of the vascular cambium of Robinia pseudoacacia. Trees 11:191–202
Farrar JJ, Evert RF (1997b) Ultrastructure of cell division in fusiform cells of the vascular cambium of Robinia pseudoacacia. Trees 11:203–215
Fritts HC (1976) Tree rings and climate. Academic Press, London
Frühmann K, Burgert I, Stanzl-Tschegg SE, Tschegg EK (2003) Fracture behaviour on the growth ring scale and cellular level of spruce (Picea abies [L.] Karst.) and beech (Fagus sylvatica L.) loaded in the TR crack propagation system. Holzforschung 57:653–660
Gottwald H (1961) Handelshölzer. Ferdinand Holzmann Verlag, Hamburg
Kübler H (1959) Die Ursache der Wachstumsspannungen und die Spannungen quer zur Faserrichtung. Holz Roh- Werkst. 17:1–9
Kübler H (1985) Growth stresses in trees and related wood properties. For Abstracts 48:131–189
Langer K, Ache P, Geiger D, Stinzing A, Arend M, Wind C, Regan S, Fromm J, Hedrich R. (2002) Poplar potassium transporters capable of controlling K+ homeostasis and K+-dependent xylogenesis. Plant J 32:997–1009
Larson PR (1995) The vascular cambium. Springer Verlag, Berlin
Maurer A, Fengel D (1990) A new process for imroving the quality and lignin staining of thin sections from wood tissue. Holzforschung 44:453–460
Mayhew JE, Newton AC (1998) The silviculture of mahogany (Swietenia macrophylla). CAB International, Wallingford
Mitscherlich G, Moll W, Künstle E, Maurer P (1966) Ertragskundlich-ökologische Untersuchungen im Rein- und Mischbestand. VI. Zuwachsbeginn und –ende, Stärkenänderung und jährlicher Durchmesserzuwachs. Allg Forst Jagdzeitung 137:72–91
Okuyama T, Yamamoto H, Yoshida M, Hattori Y, Archer RR (1994) Growth stresses in tension wood: role of microfibrils and lignification. Ann Sci For 51:291–300
Oribe Y, Funada R, Shibagaki M, Kubo T (2001) Cambial reactivation in locally heated stems of the evergreen conifer Abies sachalinensis (Schmidt) Masters. Planta 212:684–691
Sauter J (2000) Photosynthate allocation of the vascular cambium: facts and problems. In: Savidge RA, Barnett JR, Napier R (eds) Cell and molecular biology of wood formation. BIOS Scientific Publishers, Oxford, pp 71–84
Skatter S, Archer RR (2001) Residual stresses caused by growth stresses within a stem with radially varying spiral grain angle - two numerical solution approaches: 1) finite element method and 2) transfer matrix method. Wood Sci Technol 35:57–71
Sundberg B, Uggla C, Tuominen H (2000) Cambial growth and auxin gradients. In: Savidge RA, Barnett JR, Napier R (eds) Cell and molecular biology of wood formation. BIOS Scientific Publishers, Oxford, pp 160–188
Wagenführ R (2000) Holzatlas. DRW Fachbuch Verlag, Leipzig
Wodzicki TJ, Wodzicki AB (1973) Auxin stimulation of cambial activity in Pinus sylvestris L. II. Dependence on basipedal transport. Physiol Plant 29:288–292
Yamamoto H (1998) Generation mechanism of growth stresses in wood cell walls: roles of lignin deposition and cellulose microfibril during cell wall maturation. Wood Sci Technol 32:171–182
Acknowledgements
We thank the Deutsche Forschungsgemeinschaft (DFG) for financial support. We are indebted to R. Kutzner, MEL Mikroelektronik GmbH, Eching, Germany for hardware and software preparation and very helpful comments. We thank Ch. Csala, for cultivating the experimental plants. The provision of microtome sections of Swietenia spp. and Khaya spp. from the wood collection of the Federal Research Centre for Forestry and Forest Products by G. Koch is especially appreciated. We also thank the referees for the improvement of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Prof. Dr. Dr. h.c. mult. W. Liese on the occasion of his 80th birthday.
Rights and permissions
About this article
Cite this article
Dünisch, O., Rühmann, O. Kinetics of cell formation and growth stresses in the secondary xylem of Swietenia mahagoni (L.) Jacq. and Khaya ivorensis A. Chev. (Meliaceae). Wood Sci Technol 40, 49–62 (2006). https://doi.org/10.1007/s00226-005-0041-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-005-0041-x