Abstract
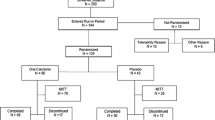
In a double-blind, placebo-controlled, randomized group comparison, new and specific biochemical markers for bone resorption as follow-up parameters on the therapeutic response to nasal salmon calcitonin (sCT) were evaluated. Evaluation took place at an outpatient clinic where osteoporosis was being researched. The subjects included 208 women aged 68–72 treated for 2 years with either 50 IU, 100 IU, or 200 IU of nasal sCT or placebo; all groups received a daily calcium supplementation of 500 mg. Only 164 women fulfilled the study as valid completers. Markers were applied to frozen urine samples of a previously published intervention study of a new fasting urinary (fU) biochemical marker for bone resorption (CrossLapsTM, ELISA) and the urinary excretion of cross-links (pyridinoline and deoxypyridinoline) was measured, all corrected for creatinine. Bone mineral density of the lumbar spine and rates of vertebral and peripheral fractures were measured after 2 years of treatment. The creatinine corrected urinary pyridinoline, deoxypyridinoline, and CrossLaps showed maximum decreases of 10–43% (95% confidence interval-29.5% to 9.6% and -75.1% to 9.3%;P < 0.01-0.001) after 6–9 months, after which the response leveled off. A significant difference among the four treatment groups was seen in fU CrossLaps(P < 0.01). The changes in spinal bone mass were significantly related to the decreases in fU CrossLaps: women with the highest response in spinal bone mass had decreases in fU CrossLaps of 44% (-83.5% to 7.4%) and women without response of 5% (-57.6% to 99.9%)P 0.001). In women who fractured during the 2-year period, fU CrossLaps remained unchanged, whereas decreases of 30% (-75.1% to 44.7%) were seen in women who did not fracture(P = 0.002). The results suggest that biochemical markers can be used to determine the optimum treatment regimen of nasal sCT. The response of the new marker, fU CrossLaps, significantly reflects the responses in bone mass of the spine and fracture rates.
Similar content being viewed by others
References
Hedlund T, Hulth A, Johnell O (1983) Early effects of parathormone and calcitonin on the number of osteoclasts and on serum-calcium in rats. Acta Orthop Scand 54:802–804
Overgaard K, Riis BJ, Christiansen C, P0denphant J, Johansen JS (1989) Nasal calcitonin for treatment of established osteoporosis. Clin Endocrinol 30:435–442
Overgaard K, Hansen MA, Jensen SB, Christiansen C (1992) Effect of salcatonin given intranasally on bone mass and fracture rates in established osteoporosis. A dose-response study. Br Med J 305:556–561
Farley JR, Hall SL, Herring S, Tarbaux NM (1992) Two biochemical indices of mouse bone formation are increased, in vivo, in response to calcitonin. Calcif Tissue Int 50:67–73
Gruber HE, Ivey JL, Baylink DJ, Matthews M, Nelp WB, Sisom K, Chesnut CH III (1984) Long-term calcitonin therapy in postmenopausal osteoporosis. Metabolism 33:295–303
Overgaard K, Agnusdei D, Hansen MA, Maioli E, Christiansen C, Gennari C (1991) Dose-response bioactivity and bioavailability of salmon calcitonin in premenopausal and postmenopausal women. J Clin Endocrinol Metab 72:344–349
Fleisch H (1991) Bisphosphonates. Pharmacology and use in the treatment of tumour-induced hypercalcaemic and metastatic bone disease. Drugs 42:919–944
Gennari C, Chierichetti SM, Bigazzi S, Fusi L, Gonnelli S, Ferrara R, Zacchei F (1985) Comparative effects of bone mineral content of calcium and calcium plus salmon calcitonin given in two different regimens in postmenopausal osteoporosis. Curr Ther Res 38:455–464
Bouizar Z, Rostene WH, Milhaud G (1987) Down-regulation of rat kidney calcitonin receptors by salmon calcitonin infusion evidenced by autoradiography. Proc Natl Acad Sei USA 84:5125–5128
Reginster JY, Denis D, Albert A, Deroisy R, Lecart MP, Fontaine MA, Lambelin P, Franchimont P (1987) 1-year controlled randomized trial of prevention of early postmenopausal bone loss by intranasal calcitonin. Lancet ii: 1481–1483
Overgaard K, Hansen MA, Herss Nielsen V-A, Riis BJ, Christiansen C (1990) Discontinuous calcitonin treatment of established osteoporosis-effects of withdrawal of treatment. Am J Med 89:1–6
Gennari C, Agnusdei D, Cepollaro C, Zacchei F, Camporeale A, Montagnani M (1992) Effect on bone mass and bone turnover of a 2-year intermittent or continuous treatment with salmon calcitonin nasal spray in postmenopausal osteoporotic patients. In: Cohn DV, Gennari C, Tashjian AH (eds) Calcium regulating hormones and bone metabolism. Elsevier Science Publishers, Netherlands, pp 400–406
Schlemmer A, Johansen JS, Pedersen SA, J0rgensen JOL, Hassager C, Christiansen C (1991) The effect of growth hormone (GH) therapy on urinary pyridinoline cross-links in GH-deficient adults. Clin Endocrinol 35:471–4176
Bonde M, Qvist P, Fledelius C, Riis BJ, Christiansen C (1994) Evaluation of an immunoassay (CrossLapsTM ELISA) for quantitation of type I collagen degradation products in urine. Clin Chem 40:2022–2025
Bonde M, Qvist P, Fledelius C, Riis BJ, Christiansen C (1995) Applications of an enzyme immunoassay for a new marker of bone resorption (CrossLaps). Follow-up on hormone replacement therapy and osteoporosis risk assessment. J Clin Endocrinol Metab 80:864–868
The Committee on Enzymes of the Scandinavian Society for Clinical Chemistry and Clinical Physiology: Recommended methods for the determination of four enzymes in blood (1974) Scand J Clin Lab Invest 33:291–306
Johansen JS, M0lholm Hansen JE, Christiansen C (1987) A radioimmunoassay for bone Gla protein (BGP) in human plasma. Acta Endocrinol 114:410–416
Podenphant J, Larsen N-E, Christiansen C (1984) An easy and reliable method for determination of urinary hydroxyproline. Clin Chim Acta 142:145–148
Melton III LJ (1988) Epidemiology of fractures. In: Riggs BL, Melton III LJ (eds). Osteoporosis. Etiology, diagnosis, and management. New York: Raven Press, pp 133–154
Matthews JNS, Altman DG, Campbell MJ, Royston P (1990) Analysis of serial measurements in medical research. Br Med J 300:230–235
Peck WA, Christiansen C, Fleisch HA, Genant HK, Gennari C, Martin TJ et al. (1993) Consensus development conference: diagnosis, prophylaxis and treatment of osteoporosis. Am J Med 94:646–650
Jordan A, Dutta S, Lutwak L, Troendle G, Sobel S (1993) Draft Version of FDA-Guidelines for preclinical and clinical evaluation of agents used in the prevention or treatment of postmenopausal osteoporosis. Division of Metabolism and Endocrine Drug Products Food and Drug Administration 1-19
Nilas L, P0denphant J, Riis BJ, Gotfredsen A, Christiansen C (1987) Usefulness of regional bone measurements in patients with osteoporotic fractures of the spine and distal forearm. J Nucl Med 28:960–965
Christiansen C, Riis BJ (1990) 17ß-Estradiol and continuous norethisterone: a unique treatment for established osteoporosis in elderly women. J Clin Endocrinol Metab 71:836–841
Munk Nielsen N, von der Recke P, Hansen MA, Overgaard K, Christiansen C (1994) Estimation of the effect of salmon calcitonin in established osteoporosis by biochemical bone markers. Calcif Tissue Int 55:8–11
Riis BJ, Overgaard K, Christiansen C (1995) Biochemical markers of bone turnover to monitor the bone response to postmenopausal hormone replacement therapy. Osteoporosis Int 5:276–280
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Overgaard, K., Christiansen, C. A new biochemical marker of bone resorption for follow-up on treatment with nasal salmon calcitonin. Calcif Tissue Int 59, 12–16 (1996). https://doi.org/10.1007/s002239900077
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s002239900077