Abstract
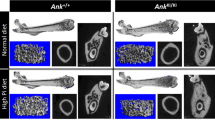
Gnathodiaphyseal dysplasia (GDD; OMIM#166260) is a rare skeletal disorder which is mainly characterized by cemento-osseous lesions in mandibles, bone fragility, bowing and diaphyseal sclerosis of tubular bones. GDD is caused by point mutations in Anoctamin-5 (ANO5); however, the disease mechanisms remain unclear. Here we generated Ano5-knockout (KO) mice using a CRISPR/Cas 9 approach to study loss of function aspects of GDD mutations. Homozygous Ano5 knockout mice (Ano5−/−) replicate some typical traits of human GDD including massive jawbones, bowing tibia, sclerosis and cortical thickening of femoral and tibial diaphyses. Serum alkaline phosphatase (ALP) levels were elevated in Ano5−/− mice as in GDD patients. Calvaria-derived Ano5−/− osteoblast cultures show increased osteoblastogenesis, which is consistent with our previous in vitro observations. Bone matrix is hypermineralized, and the expression of bone formation-related factors is enhanced in Ano5−/− mice, suggesting that the osteogenic anomaly arises from a genetic disruption of Ano5. We believe this new mouse model will shed more light on the development of skeletal abnormalities in GDD on a cellular and molecular level.






Similar content being viewed by others
References
Akasaka Y, Nakajima T, Koyama K et al (1969) Familial cases of a new systemic bone disease, hereditary gnathodiaphyseal sclerosis. Nippon Seikeigeka Gakkai Zasshi 43:381–394
Riminucci M, Collins MA, Boyde A et al (2001) Gnathodiaphyseal dysplasia: a syndrome of fibro-osseous lesions of jawbones, bone fragility, and long bone bowing. J Bone Miner Res 16:1710–1718
Ahluwalia J, Ly JQ, Norman E et al (2001) Gnathodiaphyseal dysplasia. Clin Imaging 31:67–69
Tsutsumi S, Kamata N, Maruoka Y et al (2003) Autosomal dominant gnathodiaphyseal dysplasia maps to chromosome 11p14.3–15.1. J Bone Miner Res 18:413–418
Jin L, Liu Y, Sun F et al (2017) Three novel ANO5 missense mutations in Caucasian and Chinese families and sporadic cases with gnathodiaphyseal dysplasia. Sci Rep 7:40935
Tsutsumi S, Kamata N, Vokes TJ et al (2004) The novel gene encoding a putative transmembrane protein is mutated in gnathodiaphyseal dysplasia (GDD). Am J Hum Genet 74:1255–1261
Marconi C, Brunamonti Binello P, Badiali G et al (2013) A novel missense mutation in ANO5/TMEM16E is causative for gnathodiaphyseal dysplasia in a large Italian pedigree. Eur J Hum Genet 21:613–619
Rolvien T, Koehne T, Kornak U et al (2017) A novel ANO5 mutation causing gnathodiaphyseal dysplasia with high bone turnover osteosclerosis. J Bone Miner Res 32:277–284
Vengoechea J, Carpenter L (2015) Gnathodiaphyseal dysplasia presenting as polyostotic fbrous dysplasia. Am J Med Genet A 167:1421–1422
Andreeva TV, Tyazhelova TV, Rykalina VN et al (2016) Whole exome sequencing links dental tumor to an autosomal-dominant mutation in ANO5 gene associated with gnathodiaphyseal dysplasia and muscle dystrophies. Sci Rep 6:26440
Duong HA, Le KT, Soulema AL et al (2016) Gnathodiaphyseal dysplasia: report of a family with a novel mutation of the ANO5 gene. Oral Surg Oral Med Oral Pathol Oral Radiol 121:123–128
Hartzell HC, Yu K, Xiao Q et al (2009) Anoctamin/TMEM16 family members are Ca2+-activated Cl– channels. J Physiol 587:2127–2139
Tran TT, Tobiume K, Hirono C et al (2014) TMEM16E (GDD1) exhibits protein instability and distinct characteristics in chloride channel/pore forming ability. J Cell Physiol 229:181–190
Duran C, Qu Z, Osunkoya AO et al (2012) ANOs 3–7 in the anoctamin/Tmem16 Cl– channel family are intracellular proteins. Am J Physiol Cell Physiol 302:C482–C493
Di Zanni E, Gradogna A, Scholz-Starke J et al (2018) Gain of function of TMEM16E/ANO5 scrambling activity caused by a mutation associated with gnathodiaphyseal dysplasia. Cell Mol Life Sci 75:1657–1670
Mizuta K, Tsutsumi S, Inoue H et al (2007) Molecular characterization of GDD1/TMEM16E, the gene product responsible for autosomal dominant gnathodiaphyseal dysplasia. Biochem Biophys Res Commun 357:126–132
Ehlen HW, Chinenkova M, Moser M et al (2013) Inactivation of anoctamin-6/Tmem16f, a regulator of phosphatidylserine scrambling in osteoblasts, leads to decreased mineral deposition in skeletal tissues. J Bone Miner Res 28:246–259
Ousingsawat J, Wanitchakool P, Schreiber R et al (2015) Anoctamin-6 controls bone mineralization by activating the calcium transporter NCX1. J Biol Chem 290:6270–6280
Zhao P, Torcaso A, Mariano A et al (2014) Anoctamin 6 regulates C2C12 myoblast proliferation. PLoS ONE 9:e92749
Hicks D, Sarkozy A, Muelas N et al (2011) A founder mutation in Anoctamin 5 is a major cause of limb-girdle muscular dystrophy. Brain 134:171–182
Little AA, McKeever PE, Gruis KL (2013) Novel mutations in the Anoctamin 5 gene (ANO5) associated with limb-girdle muscular dystrophy 2L. Muscle Nerve 47:287–291
Magri F, Del Bo R, D’Angelo MG et al (2012) Frequency and characterisation of anoctamin 5 mutations in a cohort of Italian limb-girdle muscular dystrophy patients. Neuromuscul Disord 22:934–943
Griffin DA, Johnson RW, Whitlock JM et al (2016) Defective membrane fusion and repair in Anoctamin5-deficient muscular dystrophy. Hum Mol Genet 25:1900–1911
Xu J, El Refaey M, Xu L et al (2015) Genetic disruption of Ano5 in mice does not recapitulate human ANO5-deficient musculardystrophy. Skelet Muscle 5:43
Gyobu S, Miyata H, Ikawa M et al (2015) A role of TMEM16E carrying a scrambling domain in sperm motility. Mol Cell Biol 36:645–659
Sui T, Xu L, Lau YS et al (2018) Development of muscular dystrophy in a CRISPR-engineered mutant rabbit model with frame-disrupting ANO5 mutations. Cell Death Dis 9:609
Otaify GA, Whyte MP, Gottesman GS et al (2018) Gnathodiaphyseal dysplasia: Severe atypical presentation with novel heterozygous mutation of the anoctamin gene (ANO5). Bone 107:167–171
Schessl J, Kress W, Schoser B (2012) Novel ANO5 mutations causing hyper-CK-emia, limb girdle muscular weakness and Miyoshi type of muscular dystrophy. Muscle Nerve 45:740–742
Bolduc V, Marlow G, Boycott KM et al (2010) Recessive mutations in the putative calcium-activated chloride channel Anoctamin 5 cause proximal LGMD2L and distal MMD3 muscular dystrophies. Am J Hum Genet 86:213–221
Witting N, Duno M, Petri H et al (2013) Anoctamin 5 muscular dystrophy in Denmark: prevalence, genotypes, phenotypes, cardiac findings, and muscle protein expression. J Neurol 260:2084–2093
Sarkozy A, Hicks D, Hudson J et al (2013) ANO5 gene analysis in a large cohort of patients with anoctaminopathy: confirmation of male prevalence and high occurrence of the common exon 5 gene mutation. Hum Mutat 34:1111–1118
Penisson-Besnier I, Saint-Andre JP, Hicks D et al (2012) Myopathy caused by anoctamin 5 mutations and necrotizing vasculitis. J Neurol 259:1988–1990
Liao BY, Zhang J (2008) Null mutations in human and mouse orthologs frequently result in different phenotypes. Proc Natl Acad Sci USA 105:6987–6992
Chipman SD, Sweet HO, McBride DJ Jr et al (1993) Defective pro alpha 2(I) collagen synthesis in a recessivemutation in mice: a model of human osteogenesis imperfecta. Proc Natl Acad Sci USA 90:1701–1705
Ueki Y, Lin CY, Senoo M et al (2007) Increased myeloid cell responses to M-CSF and RANKL cause bone loss and inflammation in SH3BP2 “cherubism” mice. Cell 128:71–83
Chen IP, Wang CJ, Strecker S (2009) Introduction of a Phe377del mutation in ANK creates a mouse model for craniometaphysealdysplasia. J Bone Miner Res 24:1206–1215
Mestas J, Hughes CCW (2004) Of mice and not men: differences between mouse and human immunology. J Immunol 172:2731–2738
Terpstra AHM (2001) Differences between humans and mice in efficacy of the body fat lowering effect of conjugated linoleic acid: role of metabolic rate. J Nutr 131:2067–2068
Wahbi K, Behin A, Becane HM (2013) Dilated cardiomyopathy in patients with mutations in anoctamin 5. Int J Cardiol 16876–16879
Campos-Xavier B, Saraiva JM, Savarirayan R et al (2001) Phenotypic variability at the TGF-beta1 locus in Camurati-Engelmann disease. Hum Genet 109:653–658
Sodek J, Ganss B, McKee MD (2000) Osteopontin. Crit Rev Oral Biol Med 11:279–303
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant # 81570958); Beijing Natural Science Foundation (Grant #7162075); High-level Talents of Beijing Health System (Grant #2013-3-036); and Scientific Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (Grant # 2015-1098).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Xiaoyu Wang, Xiu Liu, Rui Dong, Chao Liang, Ernst J. Reichenberger and Ying Hu declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
The animal experimentation protocols were approved by the Institutional Animal Care and Use Committee of the Beijing Stomatological Hospital (the approval number: KQYY-201611-001) and were strictly undertaken in accordance with the ethical guidelines of the Caring for Laboratory Animals by the Ministry of Science and Technology of the People’s Republic of China.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
223_2019_528_MOESM1_ESM.docx
Supplementary material 1. Fig. S1 Skeletal muscle changes in Ano5+/+ and Ano5-/- mice. (A) H&E staining of gastrocnemius muscle of 16-week-old mice. Scale bar = 50 μm. (B) qPCR analysis of Dysferlin and Dystrophin in gastrocnemius (GAS), quadriceps (QD) and biceps muscles of 16-week-old mice. aP < 0.05, bP < 0.01. (C) Elevated serum creatine kinase (CK) level was detected in 16-week-old Ano5-/- mice. (n = 5). bP < 0.01 (DOCX 1084 KB)
Rights and permissions
About this article
Cite this article
Wang, X., Liu, X., Dong, R. et al. Genetic Disruption of Anoctamin 5 in Mice Replicates Human Gnathodiaphyseal Dysplasia (GDD). Calcif Tissue Int 104, 679–689 (2019). https://doi.org/10.1007/s00223-019-00528-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-019-00528-x