Abstract
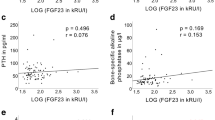
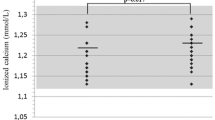
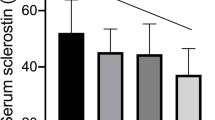
We tested the hypothesis that the levels of bone remodeling mediators may be altered in Prader–Willi syndrome (PWS). We assessed RANKL, OPG, sclerostin, DKK-1 serum levels, and bone metabolism markers in 12 PWS children (7.8 ± 4.3 years), 14 PWS adults (29.5 ± 7.2 years), and 31 healthy controls matched for sex and age. Instrumental parameters of bone mineral density (BMD) were also evaluated. Lumbar spine BMD Z-scores were reduced in PWS children (P < 0.01), reaching osteopenic levels in PWS adults. PWS patients showed lower 25(OH)-vitamin D serum levels than controls (P < 0.001). Osteocalcin was increased in PWS children but reduced in adults respect to controls (P < 0.005 and P < 0.01, respectively). RANKL levels were higher in both pediatric and PWS adults than controls (P < 0.004), while OPG levels were significantly reduced (P < 0.004 and P < 0.006, respectively). Sclerostin levels were increased in children (P < 0.04) but reduced in adults compared to controls (P < 0.01). DKK-1 levels did not show significant difference between patients and controls. In PWS patients, RANKL, OPG, and sclerostin significantly correlated with metabolic and bone instrumental parameters. Consistently, with adjustment for age, multiple linear regression analysis showed that BMD and osteocalcin were the most important predictors for RANKL, OPG, and sclerostin in children, and GH and sex steroid replacement treatment in PWS adults. We demonstrated the involvement of RANKL, OPG, and sclerostin in the altered bone turnover of PWS subjects suggesting these molecules as markers of bone disease and new potential pharmacological targets to improve bone health in PWS.
Similar content being viewed by others
References
Butler MG, Manzardo AM, Forster JL (2016) Prader-Willi syndrome: clinical genetics and diagnostic aspects with treatment approaches. Curr Pediatr Rev 12:136–166
Lionti T, Reid SM, White SM, Rowell MM (2015) A population-based profile of 160 Australians with Prader-Willi syndrome: trends in diagnosis, birth prevalence and birth characteristics. Am J Med Genet A 167A:371–378
Cassidy SB, Schwartz S, Miller JL, Driscoll DJ (2012) Prader-Willi syndrome. Genet Med 14:10–26
Faienza MF, Ventura A, Lauciello R et al (2012) Analysis of endothelial protein C receptor gene and metabolic profile in Prader-Willi syndrome and obese subjects. Obesity 20:1866–1870
Angulo MA, Butler MG, Cataletto ME (2015) Prader-Willi syndrome: a review of clinical, genetic, and endocrine findings. J Endocrinol Invest 38:1249–1263
Burnett LC, LeDuc CA, Sulsona CR et al (2016) Deficiency in prohormone convertase PC1 impairs prohormone processing in Prader-Willi syndrome. J Clin Invest. https://doi.org/10.1172/JCI88648
Vestergaard P, Kristensen K, Bruun JM, Østergaard JR, Heickendorff L, Mosekilde L, Richelsen B (2004) Reduced bone mineral density and increased bone turnover in Prader-Willi syndrome compared with controls matched for sex and body mass index: a cross-sectional study. J Pediatr 144:614–619
Longhi S, Grugni G, Gatti D et al (2015) Adults with Prader-Willi syndrome have weaker bones: effect of treatment with GH and sex steroids. Calcif Tissue Int 96:160–166
Butler MG, Haber L, Mernaugh R, Carlson MG, Price R, Feurer ID (2001) Decreased bone mineral density in Prader-Willi syndrome: comparison with obese subjects. Am J Med Genet 103:216–222
Kroonen LT, Herman M, Pizzutillo PD, Macewen GD (2006) Prader-Willi syndrome: clinical concerns for the orthopaedic surgeon. J Pediatr Orthop 26:673–679
Sinnema M, Maaskant MA, van Schrojenstein Lantman-de Valk HM, van Nieuwpoort IC, Drent ML, Curfs LM, Schrander-Stumpel CT (2011) Physical health problems in adults with Prader-Willi syndrome. Am J Med Genet A 155A:2112–2124
Carrel AL, Myers SE, Whitman BY, Allen DB (2002) Benefits of long-term GH therapy in Prader-Willi syndrome: a 4-year study. J Clin Endocrinol Metab 87:1581–1585
Carrel AL, Myers SE, Whitman BY, Eickhoff J, Allen DB (2010) Long-term growth hormone therapy changes the natural history of body composition and motor function in children with Prader-Willi syndrome. J Clin Endocrinol Metab 95:1131–1136
de Lind van Wijngaarden RF, Festen DA, Otten BJ et al (2009) Bone mineral density and effects of growth hormone treatment in prepubertal children with Prader-Willi syndrome: a randomized controlled trial. J Clin Endocrinol Metab 94:3763–3771
Jørgensen AP, Ueland T, Sode-Carlsen R et al (2013) Two years of growth hormone treatment in adults with Prader-Willi syndrome do not improve the low BMD. J Clin Endocrinol Metab 98:E753–E760
Grugni G (2013) Prader-Willi syndrome: GH therapy and bone. Nat Rev Endocrinol 9:320–321
Khor EC, Fanshawe B, Qi Y et al (2016) Prader-Willi critical region, a non-translated, imprinted central regulator of bone mass: possible role in skeletal abnormalities in Prader-Willi syndrome. PLoS ONE 11:e0148155
Baron R, Kneissel M (2013) WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19:1791–1792
Qiang YW, Chen Y, Stephens O et al (2008) Myeloma-derived Dickkopf-1 disrupts Wnt-regulated osteoprotegerin and RANKL production by osteoblasts: a potential mechanism underlying osteolytic bone lesions in multiple myeloma. Blood 112:196–207
Lewiecki EM (2014) Role of sclerostin in bone and cartilage and its potential as a therapeutic target in bone diseases. Ther Adv Musculoskelet Dis 6:48–57
Holm VA, Cassidy SB, Butler MG, Hanchett JM, Greenswag LR, Whitman BY, Greenberg F (1993) Prader-Willi syndrome: consensus diagnostic criteria. Pediatrics 91:398–402
Corneli G, Di Somma C, Baldelli R et al (2005) The cut-off limits of the GH response to GH-releasing hormone-arginine test related to body mass index. Eur J Endocrinol 153:257–264
Cole TJ, Bellizzi MC, Flegal KM, Dietz WH (2000) Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320:1240–1243
Kuczmarski RJ, Ogden CL, Grummer-Strawn LM et al (2000) CDC growth charts: United States. Adv Data 8:1–27
Tanner JM, Whitehouse RH (1976) Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child 51:170–179
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Vignali DA (2000) Multiplexed particle-based flow cytometric assays. J Immunol Methods 243:243–255
Salle BL, Braillon P, Glorieux FH, Brunet J, Cavero E, Meunier PJ (1992) Lumbar bone mineral content measured by dual energy X-ray absorptiometry in newborns and infants. Acta Paediatr 81:953–958
Southard RN, Morris JD, Mahan JD, Hayes JR, Torch MA, Sommer A, Zipf WB (1991) Bone mass in healthy children: measurement with quantitative DXA. Radiology 179:735–738
Glastre C, Braillon P, David L, Cochat P, Meunier PJ, Delmas PD (1990) Measurement of bone mineral content of the lumbar spine by dual energy X-ray absorptiometry in normal children: correlations with growth parameters. J Clin Endocrinol Metab 70:1330–1333
Faienza MF, Brunetti G, Colucci S et al (2009) Osteoclastogenesis in children with 21-hydroxylase deficiency on long-term glucocorticoid therapy: the role of receptor activator of nuclear factor-kappaB ligand/osteoprotegerin imbalance. J Clin Endocrinol Metab 94:2269–2276
Faienza MF, Brunetti G, Ventura A et al (2015) Mechanisms of enhanced osteoclastogenesis in girls and young women with Turner’s Syndrome. Bone 81:228–236
Brunetti G, Papadia F, Tummolo A et al (2016) Impaired bone remodeling in children with osteogenesis imperfecta treated and untreated with bisphosphonates: the role of DKK1, RANKL, and TNF-α. Osteoporos Int 27:2355–2365
Faienza MF, Ventura A, Delvecchio M et al (2017) High sclerostin and Dickkopf-1 (DKK-1) serum levels in children and adolescents with type 1 diabetes mellitus. J Clin Endocrinol Metab 102:1174–1181
Giordano P, Brunetti G, Lassandro G et al (2016) High serum sclerostin levels in children with haemophilia A. Br J Haematol 172:293–295
Delgado-Calle J, Bellido T (2015) Osteocytes and skeletal pathophysiology. Curr Mol Biol Rep 1(4):157–167
Mogul HR, Lee PD, Whitman BY et al (2008) Growth hormone treatment of adults with Prader-Willi syndrome and growth hormone deficiency improves lean body mass, fractional body fat, and serum triiodothyronine without glucose impairment: results from the United States multicenter trial. J Clin Endocrinol Metab 93:1238–1245
Sode-Carlsen R, Farholt S, Rabben KF et al (2012) Growth hormone treatment in adults with Prader-Willi syndrome: the Scandinavian study. Endocrine 41:191–199
Sanchez-Ortiga R, Klibanski A, Tritos NA (2012) Effects of recombinant human growth hormone therapy in adults with Prader-Willi syndrome: a meta-analysis. Clin Endocrinol 77:86–93
Tritos NA, Klibanski A (2016) Effects of growth hormone on bone. Prog Mol Biol Transl Sci 138:193–211
Bakker NE, Kuppens RJ, Siemensma EP et al (2015) Bone mineral density in children and adolescents with Prader-Willi syndrome: longitudinal study during puberty and 9 years of growth hormone treatment. J Clin Endocrinol Metab 100:1609–1618
Nakamura Y, Murakami N, Iida T, Asano S, Ozeki S, Nagai T (2014) Growth hormone treatment for osteoporosis in patients with scoliosis of Prader-Willi syndrome. J Orthop Sci 19:877–882
Canalis E (2018) Management of endocrine disease: novel anabolic treatments for osteoporosis. Eur J Endocrinol 178(2):R33–R44
Doyon A, Fischer DC, Bayazit AK et al (2015) Markers of bone metabolism are affected by renal function and growth hormone therapy in children with chronic kidney disease. PLoS ONE 10:e0113482
Ueland T (2005) GH/IGF-I and bone resorption in vivo and in vitro. Eur J Endocrinol 152:327–332
Faienza MF, Chiarito M, D’amato G, Colaianni G, Colucci S, Grano M, Brunetti G. Monoclonal antibodies for treating osteoporosis. Expert Opin Biol Ther 2017:1–9. https://doi.org/10.1080/14712598.2018.1401607
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
G. Brunetti, G. Grugni, L. Piacente, M. Delvecchio, A. Ventura, P. Giordano, M. Grano, G. D’Amato, D. Laforgia, A. Crinò, and M. F. Faienza declare that they have no conflict of interest to disclose.
Human and Animal Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Written informed consent was obtained from all the legal guardians, and from the patients when applicable, prior to inclusion. All procedures were approved by local institutional review boards.
Rights and permissions
About this article
Cite this article
Brunetti, G., Grugni, G., Piacente, L. et al. Analysis of Circulating Mediators of Bone Remodeling in Prader–Willi Syndrome. Calcif Tissue Int 102, 635–643 (2018). https://doi.org/10.1007/s00223-017-0376-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-017-0376-y