Abstract
The central nervous system is widely known to exert control over our systemic physiology via several mechanisms including the regulation of skeletal metabolism. Neuronal circuits within the hypothalamus have been shown to impact bone mass via leptin-dependent and independent mechanisms; however, the full extent to which the brain controls bone homeostasis is not known. We previously identified cell adhesion molecule1 (Cadm1) as a regulator of body weight and energy homeostasis via its expression in multiple regions of the brain. Here, we show that loss of Cadm1 expression in excitatory neurons results in increased leptin sensitivity in addition to a concomitant reduction in bone mass. Femoral length, bone mineral content, diaphyseal cross-sectional area, and bone strength were all lower in Cadm1-deficient animals. Conversely, inducing expression of Cadm1 in excitatory neurons decreased leptin sensitivity and increased femoral length, bone mineral content, and diaphyseal cross-sectional area. Together, these results illustrate an essential role for this synaptic protein in the neuronal regulation of skeletal bone metabolism.



Similar content being viewed by others
References
Wee NKY, Kulkarni RN, Horsnell H, Baldock PA (2016) The brain in bone and fuel metabolism. Bone 82:56–63. https://doi.org/10.1016/j.bone.2015.10.020
Riddle RC, Clemens TL (2017) bone cell bioenergetics and skeletal energy homeostasis. Physiol Rev 97:667–698. https://doi.org/10.1152/physrev.00022.2016
Clemens TL, Karsenty G (2011) The osteoblast: an insulin target cell controlling glucose homeostasis. J Bone Miner Res 26:677–680. https://doi.org/10.1002/jbmr.321
Ferron M, Wei J, Yoshizawa T et al (2010) Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 142:296–308. https://doi.org/10.1016/j.cell.2010.06.003
Ducy P (2011) The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia 54:1291–1297. https://doi.org/10.1007/s00125-011-2155-z
Fulzele K, Clemens TL (2012) Novel functions for insulin in bone. Bone 50:452–456. https://doi.org/10.1016/j.bone.2011.06.018
Sato S, Hanada R, Kimura A et al (2007) Central control of bone remodeling by neuromedin U. Nat Med 13:1234–1240. https://doi.org/10.1038/nm1640
Yadav VK, Oury F, Suda N et al (2009) A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell 138:976–989. https://doi.org/10.1016/j.cell.2009.06.051
Ducy P, Amling M, Takeda S et al (2000) Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100:197–207. https://doi.org/10.1016/S0092-8674(00)81558-5
Rathjen T, Yan X, Kononenko NL et al (2017) Regulation of body weight and energy homeostasis by neuronal cell adhesion molecule 1. Nat Neurosci. https://doi.org/10.1038/nn.4590
Nakamura S, Koyama T, Izawa N et al (2017) Negative feedback loop of bone resorption by NFATc1-dependent induction of Cadm1. PLoS ONE 12:e0175632. https://doi.org/10.1371/journal.pone.0175632
Harno E, Cottrell EC, White A (2013) Metabolic pitfalls of CNS Cre-based technology. Cell Metab 18:21–28. https://doi.org/10.1016/j.cmet.2013.05.019
Bouxsein ML, Boyd SK, Christiansen BA et al (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25:1468–1486. https://doi.org/10.1002/jbmr.141
Lodberg A, Vegger JB, Jensen MV et al (2015) Immobilization induced osteopenia is strain specific in mice. Bone Rep 2:59–67. https://doi.org/10.1016/j.bonr.2015.04.001
Mosekilde L, Thomsen JS, Orhii PB et al (1999) Additive effect of voluntary exercise and growth hormone treatment on bone strength assessed at four different skeletal sites in an aged rat model. Bone 24:71–80. https://doi.org/10.1016/S8756-3282(98)00169-0
Tattikota SG, Rathjen T, McAnulty SJ et al (2014) Argonaute2 mediates compensatory expansion of the pancreatic β cell. Cell Metab 19:122–134. https://doi.org/10.1016/j.cmet.2013.11.015
Tattikota SG, Sury MD, Rathjen T et al (2013) Argonaute2 regulates the pancreatic β-cell secretome. Mol Cell Proteom 12:1214–1225. https://doi.org/10.1074/mcp.M112.024786
Upadhyay J, Farr OM, Mantzoros CS (2015) The role of leptin in regulating bone metabolism. Metabolism 64:105–113. https://doi.org/10.1016/j.metabol.2014.10.021
Turner RT, Kalra SP, Wong CP et al (2013) Peripheral leptin regulates bone formation. J Bone Miner Res 28:22–34. https://doi.org/10.1002/jbmr.1734
Fajardo RJ, Karim L, Calley VI, Bouxsein ML (2014) A review of rodent models of type 2 diabetic skeletal fragility. J Bone Miner Res 29:1025–1040. https://doi.org/10.1002/jbmr.2210
Wei J, Ducy P (2010) Co-dependence of bone and energy metabolisms. Arch Biochem Biophys 503:35–40. https://doi.org/10.1016/j.abb.2010.05.021
Katsnelson A (2010) Physiology: the bones of contention. Nat News 466:914–915. https://doi.org/10.1038/466914a
Takamori S, Rhee JS, Rosenmund C, Jahn R (2001) Identification of differentiation-associated brain-specific phosphate transporter as a second vesicular glutamate transporter (VGLUT2). J Neurosci 21:RC182
Cao JJ (2011) Effects of obesity on bone metabolism. J Orthop Surg 6:30. https://doi.org/10.1186/1749-799X-6-30
Shapses SA, Sukumar D (2012) Bone metabolism in obesity and weight loss. Annu Rev Nutr 32:287–309. https://doi.org/10.1146/annurev.nutr.012809.104655
Dimitri P, Bishop N, Walsh JS, Eastell R (2012) Obesity is a risk factor for fracture in children but is protective against fracture in adults: a paradox. Bone 50:457–466. https://doi.org/10.1016/j.bone.2011.05.011
Acknowledgements
The authors are grateful for the excellent technical assistance of Jytte Utoft (Aarhus University). This work was funded by the Helmholtz Gemeinschaft, the Helmholtz Metabolic Dysfunction Consortium, and the European Foundation for the Study of Diabetes (EFSD, Germany), and the Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD, Exc 229 to N.L.K.). The µCT scanner was kindly donated by the VELUX Foundation (Søborg, Denmark).
Author information
Authors and Affiliations
Contributions
MNP conceived this study. XY, NLK, AB, JST, and MNP designed and performed the experiments. MNP and JST wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
All animal research protocols were approved by the Landesamt für Gesundheit und Soziales Berlin (LAGeSo). This article does not contain any studies with human participants performed by any of the authors.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
223_2017_361_MOESM1_ESM.pdf
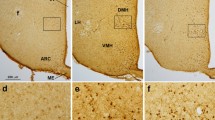
Supplementary Fig. 1: Loss of CADM1 in the brain of Slc17a6-Cre, Cadm1 flox/flox mice. (A) Double immunostaining of Cadm1 (green) and VGLUT2 (red) identifies co-localization of Cadm1 and VGLUT2 (yellow) in the dentate gyrus of the hippocampus. (B) Double immunostaining of Cadm1 (green) and VGLUT2 (red) identifies co-localization of Cadm1 and VGLUT2 (yellow) in the medial habenula. Dotted white boxes indicate the areas magnified to the left. Supplementary material 1 (PDF 2986 kb)
Rights and permissions
About this article
Cite this article
Yan, X., Kononenko, N.L., Brüel, A. et al. Neuronal Cell Adhesion Molecule 1 Regulates Leptin Sensitivity and Bone Mass. Calcif Tissue Int 102, 329–336 (2018). https://doi.org/10.1007/s00223-017-0361-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-017-0361-5