Abstract
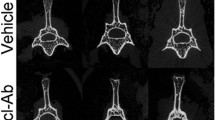
The inhibition of sclerostin by the systemic administration of a monoclonal antibody (Scl-Ab) significantly increased bone mass and strength in fractured bones in animal models and non-fractured bones in ovariectomised (OVX) rats. In this study, the effects of Scl-Ab on healing were examined in a closed fracture model in OVX rats. Sixty Sprague-Dawley rats underwent an ovariectomy or a sham operation at 4 months of age, and a closed fracture of the right femur was performed 3 months later. Subcutaneous injections with Scl-Ab (25 mg/kg) or saline were then administered on day 1 after the fracture and twice a week for 8 weeks (n = 20 per group), at which time the fractured femurs were harvested for micro-computed tomography analysis, four-point bending mechanical testing and histomorphometric analysis to examine bone mass, bone strength and dynamic bone formation at the fracture site. The angiogenesis at the fracture site was also examined. Bone marrow stem cells were also isolated from the fractured bone to perform a colony-forming unit (CFU) assay and an alkaline phosphatase-positive (ALP+) CFU assay. OVX rats treated with Scl-Ab for 8 weeks had significantly increased bone mineral density and relative bone volume compared with OVX rats treated with saline. Similarly, maximum loading, energy to maximum load and stiffness in Scl-Ab-treated OVX rats were significantly higher than those in saline controls. The mineral apposition rate (MAR), mineralising surface (MS/BS) and bone formation rate (BFR/BS) were also significantly increased in Scl-Ab-treated group compared with the saline-treated group in OVX rats. Furthermore, the Scl-Ab-treated group had more CFUs and ALP+ CFUs than the saline-treated group in OVX rats. No significant difference in angiogenesis at the fracture site was found between the groups. Our study demonstrated that Scl-Ab helped to increase bone mass, bone strength and bone formation at the fracture site in a closed femoral fracture model in OVX rats. Bone marrow stem cells in OVX rats injected with Scl-Ab also had increased CFUs and ALP+ CFUs.





Similar content being viewed by others
References
Cummings SR, Melton LJ (2002) Epidemiology and outcomes of osteoporotic fractures. Lancet 359(9319):1761–1767
Johnell O, Kanis J (2005) Epidemiology of osteoporotic fractures. Osteoporos Int 16(Suppl 2):S3–S7
Ke HZ et al (2012) Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev 33(5):747–783
McKibbin B (1978) The biology of fracture healing in long bones. J Bone Joint Surg Br 60-B(2):150–162
Frost HM (1989) The biology of fracture healing. An overview for clinicians. Part I. Clin Orthop Relat Res 248:283–293
Li X et al (2009) Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res 24(4):578–588
Ring D et al (2004) Locking compression plates for osteoporotic nonunions of the diaphyseal humerus. Clin Orthop Relat Res 425:50–54
Hill TP et al (2005) Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell 8(5):727–738
Hoeppner LH, Secreto FJ, Westendorf JJ (2009) Wnt signaling as a therapeutic target for bone diseases. Expert Opin Ther Targets 13(4):485–496
Komatsu DE et al (2010) Modulation of Wnt signaling influences fracture repair. J Orthop Res 28(7):928–936
Zhang R et al (2013) Wnt/beta-catenin signaling activates bone morphogenetic protein 2 expression in osteoblasts. Bone 52(1):145–156
Kamiya N et al (2008) BMP signaling negatively regulates bone mass through sclerostin by inhibiting the canonical Wnt pathway. Development 135(22):3801–3811
Krishnan V, Bryant HU, Macdougald OA (2006) Regulation of bone mass by Wnt signaling. J Clin Invest 116(5):1202–1209
Ten Dijke P et al (2008) Osteocyte-derived sclerostin inhibits bone formation: its role in bone morphogenetic protein and Wnt signaling. J Bone Joint Surg Am 90(Suppl 1):31–35
Van Bezooijen RL et al (2007) Wnt but not BMP signaling is involved in the inhibitory action of sclerostin on BMP-stimulated bone formation. J Bone Miner Res 22(1):19–28
Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR (2001) Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet 68:577–589
Balemans W, Ebeling M, Patel N (2001) Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet 10(5):537–543
Kramer I et al (2010) Parathyroid hormone (PTH)-induced bone gain is blunted in SOST overexpressing and deficient mice. J Bone Miner Res 25(2):178–189
McDonald MM et al (2012) Inhibition of sclerostin by systemic treatment with sclerostin antibody enhances healing of proximal tibial defects in ovariectomized rats. J Orthop Res 30(10):1541–1548
Padhi D et al (2011) Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res 26(1):19–26
Bonnarens Frank, Einhorn TA (1984) Production of a standard closed fracture in laboratory animal bone. J Orthop Res 2(1):97–102
Mittra E et al (2008) Evaluation of trabecular mechanical and microstructural properties in human calcaneal bone of advanced age using mechanical testing, microCT, and DXA. J Biomech 41(2):368–375
Suen PK et al (2014) Sclerostin monoclonal antibody enhanced bone fracture healing in an open osteotomy model in rats. J Orthop Res 32(8):997–1005
Dempster DW et al (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 28(1):2–17
He YX et al (2011) Impaired bone healing pattern in mice with ovariectomy-induced osteoporosis: a drill-hole defect model. Bone 48(6):1388–1400
He YX et al (2012) Deletion of estrogen receptor beta accelerates early stage of bone healing in a mouse osteotomy model. Osteoporos Int 23(1):377–389
Xu L et al (2012) Cellular retinol-binding protein 1 (CRBP-1) regulates osteogenenesis and adipogenesis of mesenchymal stem cells through inhibiting RXRalpha-induced beta-catenin degradation. Int J Biochem Cell Biol 44(4):612–619
Augat P et al (2005) Mechanics and mechano-biology of fracture healing in normal and osteoporotic bone. Osteoporos Int 16(Suppl 2):S36–S43
Ominsky MS et al (2011) Inhibition of sclerostin by monoclonal antibody enhances bone healing and improves bone density and strength of nonfractured bones. J Bone Miner Res 26(5):1012–1021
Padhi D, Stouch B, Jang G, Fang L, Darling M, Glise H, Robinson M, Harris S, Posvar E (2007) Anti-sclerostin antibody increases markers of bone formation in healthy postmenopausal women. J Bone Miner Res S1:S37
Hamann C et al (2013) Sclerostin antibody treatment improves bone mass, bone strength, and bone defect regeneration in rats with type 2 diabetes mellitus. J Bone Miner Res 28(3):627–638
Saito M et al (2010) Comparison of effects of alfacalcidol and alendronate on mechanical properties and bone collagen cross-links of callus in the fracture repair rat model. Bone 46(4):1170–1179
Center JR, Bliuc D, Nguyen TV, Eisman JA (2007) Risk of subsequent fracture after low trauma fracture in men and women. JAMA 297(4):387–394
Heino TJ, Hentunen TA (2008) Differentiation of osteoblasts and osteocytes from mesenchymal stem cells. Curr Stem Cell Res Ther 3(2):131–145
Farley JR, Baylink DJ (1986) Skeletal alkaline phosphatase activity as a bone formation index in vitro. Metabolism 35(6):563–572
Sutherland MK et al (2004) Sclerostin promotes the apoptosis of human osteoblastic cells: a novel regulation of bone formation. Bone 35(4):828–835
Winkler DG, Sutherland MK, Geoghegan JC (2003) Osteocyte control of bone formation via sclerostin. EMBO J 22(23):6267–6276
Jacobsen KA et al (2008) Bone formation during distraction osteogenesis is dependent on both VEGFR1 and VEGFR2 signaling. J Bone Miner Res 23(5):596–609
Street J et al (2002) Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA 99(15):9656–9661
Lu C, Marcucio R, Miclau T (2006) Assessing angiogenesis during fracture healing. Iowa Orthop J 26:17–26
Acknowledgments
We thank Amgen Inc. and UCB Pharma who provided funding and Scl-Ab for this study. This work was supported by a Grant from the Hong Kong Government Research Grant Council, General Research Fund (CUHK470813) and a Grant from China Shenzhen City Science and Technology Bureau under the Shenzhen City Knowledge Innovation Plan, Basic Research Project (JCYJ20130401171935811) to Gang Li. This study was also supported in part by the SMART program, Lui Che Woo Institute of Innovative Medicine, Faculty of Medicine, The Chinese University of Hong Kong. This research project was made possible by resources donated by Lui Che Woo Foundation Limited.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
Yang Liu, Yunfeng Rui, Tin Yan Cheng, Shuo Huang, Liangliang Xu, Fanbiao Meng, Wayne Yuk Wai Lee, Ting Zhang, Nan Li, Chaoyang Li, Huazhu Ke and Gang Li declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All animal experiments were approved by the Animal Experimentation Ethics Committee of The Chinese University of Hong Kong, Hong Kong, China.
Additional information
Yang Liu and Yunfeng Rui contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, Y., Rui, Y., Cheng, T.Y. et al. Effects of Sclerostin Antibody on the Healing of Femoral Fractures in Ovariectomised Rats. Calcif Tissue Int 98, 263–274 (2016). https://doi.org/10.1007/s00223-015-0085-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-015-0085-3