Abstract
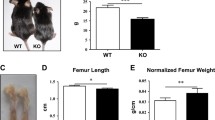
Transforming growth factor-β-induced gene product-h3 (TGFBI/BIGH3) is an extracellular matrix protein expressed in a wide variety of tissues. TGFBI binds to type I, II, and IV collagens, as well as to biglycan and decorin and plays important roles in cell-to-cell, cell-to-collagen, and cell-to-matrix interactions. Furthermore, TGFBI is involved in cell growth and migration, tumorigenesis, wound healing, and apoptosis. To investigate whether TGFBI is involved in the maintenance of skeletal tissues, Tgfbi knockout mice were generated by crossing male and female Tgfbi heterozygous mice. Skeletal preparation showed that the skeletal size in Tgfbi knockout mice was smaller than in wild-type and heterozygous mice. However, chondrocytic cell alignment in the growth plates, bone mineral density, and bone forming rates were similar in Tgfbi knockout, wild-type, and heterozygous mice. Alterations in skeletal tissue arrangements in Tgfbi knockout mice were estimated from safranin O staining, trichrome staining, and immunohistochemistry for type II and X collagen, and matrix metalloproteinase 13 (MMP13). Cartilage matrix degradation was observed in the articular cartilage of Tgfbi knockout mice. Although the detection of type II collagen in the articular cartilage was lower in Tgfbi knockout mice than wild-type mice, the detection of MMP13 was markedly higher, indicating that Tgfbi deficiency is associated with the degradation of cartilage matrix. These results suggest that TGFBI plays an important role in maintaining skeletal tissues and the cartilage matrix in mice.




Similar content being viewed by others
References
Skonier J, Neubauer M, Madisen L, Bennett K, Plowman GD, Purchio AF (1992) cDNA cloning and sequence analysis of betaig-h3, a novel gene induced in a human adenocarcinoma cell line after treatment with transforming growth factor-beta. DNA Cell Biol 11:511–522
Kawamoto T, Noshiro M, Shen M, Nakamasu K, Hashimoto K, Kawashima-Ohya Y, Gotoh O, Kato Y (1998) Structural and phylogenetic analyses of RGD-CAP/beta ig-h3, a fasciclin-like adhesion protein expressed in chick chondrocytes. Biochim Biophys Acta 1395:288–292
Skonier J, Benenett K, Rothwell V, Kosowski S, Plowman G, Wallace P, Edelhoff S, Disteche C, Neubauer M, Marquardt H, Rodgers J, Purchio AF (1994) beta ig-h3: a transforming growth factor-beta-responsive gene encoding a secreted protein that inhibits cell attachment in vitro and suppresses the growth of CHO cells in nude mice. DNA Cell Biol 13:571–584
Ma C, Rong Y, Radiloff DR, Datto MB, Centeno B, Bao S, Cheng AW, Lin F, Jiang S, Yeatman TJ, Wang XF (2008) Extracellular matrix protein beta ig-h3/TGFBI promotes metastasis of colon cancer by enhancing cell extravasation. Genes Dev 22:308–321
Rawe IM, Zhan Q, Burrows R, Bennett K, Cintron C (1997) Beta-ig. Molecular cloning and in situ hybridization in corneal tissues. Invest Ophthalmol Vis Sci 38:893–900
Kim JE, Kim SJ, Jeong HW, Lee BH, Choi JY, Park RW, Park JY, Kim IS (2003) RGD peptides released from beta ig-h3, a TGF-beta-induced cell-adhesive molecule, mediate apoptosis. Oncogene 22:2045–2053
LeBaron RG, Bezverkov KI, Zimber MP, Pavele R, Skonier J, Purchio AF (1995) Beta IGH3, a novel secretory protein inducible by transforming growth factor-beta, is present in normal skin and promotes the adhesion and spreading of dermal fibroblasts in vitro. J Invest Dermatol 104:844–849
Ohno S, Noshiro M, Makihira S, Kawamoto T, Shen M, Yan W, Kawashima-Ohya Y, Fujimoto K, Tanne K, Kato Y (1999) RGD-CAP ((beta)ig-h3) enhances the spreading of chondrocytes and fibroblasts via integrin alpha(1)beta(1). Biochim Biophys Acta 1451:196–205
Kim JE, Kim SJ, Lee BH, Park RW, Kim KS, Kim IS (2000) Identification of motifs for cell adhesion within the repeated domains of transforming growth factor-β-induced gene, βig-h3. J Biol Chem 275:30907–30915
Schorderet DF, Menasche M, Morand S, Bonnel S, Buchillier V, Marchant D, Auderset K, Bonny C, Abitbol M, Munier FL (2000) Genomic characterization and embryonic expression of the mouse Bigh3 (Tgfbi) gene. Biochem Biophys Res Commun 274:267–274
Han MS, Kim JE, Shin HI, Kim IS (2008) Expression patterns of βig-h3 in chondrocyte differentiation during endochondral ossification. Exp Mol Med 40:453–460
Thorp BH, Anderson I, Jakowlew SB (1992) Transforming growth factor-beta 1, -beta 2 and -beta 3 in cartilage and bone cells during endochondral ossification in the chick. Development 114:907–911
Blaney Davidson EN, van der Kraan PM, van den Berg WB (2007) TGF-beta and osteoarthritis. Osteoarthr Cartil 15:597–604
Yang X, Chen L, Xu X, Li C, Huang C, Deng CX (2001) TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol 153:35–46
Tang SY, Alliston T (2013) Regulation of postnatal bone homeostasis by TGF-β. Bonekey Rep 255:1–5
Kim JE, Park RW, Choi JY, Bae YC, Kim KS, Joo CK, Kim IS (2002) Molecular properties of wild-type and mutant βIG-H3 proteins. Invest Ophthalmol Vis Sci 43:656–661
Bae JS, Lee W, Nam JO, Kim JE, Kim SW, Kim IS (2014) Transforming growth factor-β-induced protein promotes severe vascular inflammatory responses. Am J Resp Crit Care 189:779–786
Baek WY, Lee MA, Jung JW, Kim SY, Akiyama H, de Crombrugghe B, Kim JE (2009) Positive regulation of adult bone formation by osteoblast-specific transcription factor Osterix. J Bone Miner Res 24:1055–1065
Baek WY, de Crombrugghe B, Kim JE (2010) Postnatally induced inactivation of Osterix in osteoblasts results in the reduction of bone formation and maintenance. Bone 46:920–928
Erben RG (1997) Embedding of bone samples in methylmethacrylate: an improved method suitable for bone histomorphometry, histochemistry, and immunohistochemistry. J Histochem Cytochem 45:307–313
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610
Bancroft JD, Layton C (2012) Connective and mesenchymal tissue with their stains. In: Suvarna SK, Layton C, Bancroft JD (eds) Bancroft’s theory and practice of histological techniques. Churchill Livingstone Elsevier, Oxford, pp 187–214
Yu H, Yang X, Cheng J, Wang X, Shen SG (2011) Distraction osteogenesis combined with tissue-engineered cartilage in the reconstruction of condylar osteochondral defect. J Oral Maxillofac Surg 69:e558–e564
Shimizu S, Asou Y, Itoh S, Chung UI, Kawaguchi H, Shinomiya K, Muneta T (2007) Prevention of cartilage destruction with intraarticular osteoclastogenesis inhibitory factor/osteoprotegerin in a murine model of osteoarthritis. Arthritis Rheum 56:3358–3365
Estrada LE, Dodge GR, Richardson DW, Farole A, Jimenez SA (2001) Characterization of a biomaterial with cartilage-like properties expressing type X collagen generated in vitro using neonatal porcine articular and growth plate chondrocytes. Osteoarthr Cartil 9:169–177
Mankin HJ, Dorfman H, Lippiello L, Zarins A (1971) Biochemical and metabolic abnormalities in articular cartilage from osteoarthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am 53:523–537
Aigner T, Cook JL, Gerwin N, Glasson SS, Laverty S, Little CB, McIlwraith W, Kraus VB (2010) Histopathology atlas of animal model systems—overview of guiding principles. Osteoarthr Cartil 18(Suppl 3):S2–S6
Kamekura S, Hoshi K, Shimoaka T, Chung U, Chikuda H, Yamada T, Uchida M, Ogata N, Seichi A, Nakamura K, Kawaguchi H (2005) Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthr Cartil 13:632–641
Chen B, Qin J, Wang H, Magdalou J, Chen L (2010) Effects of adenovirus-mediated bFGF, IL-1Ra and IGF-1 gene transfer on human osteoarthritic chondrocytes and osteoarthritis in rabbits. Exp Mol Med 42:684–695
Eyre DR, Weis MA, Wu JJ (2006) Articular cartilage collagen: an irreplaceable framework? Eur Cell Mater 12:57–63
Roberts AB, Mccune BK, Sporn MB (1992) TGF-β: regulation of extracellular matrix. Kidney Int 41:557–559
Wong M, Carter DR (2003) Articular cartilage functional histomorphology and mechanobiology: a research perspective. Bone 33:1–13
Munger JS, Sheppard D (2011) Cross talk among TGF-β signaling pathways, integrins, and the extracellular matrix. Cold Spring Harb Perspect Biol 3:a005017
Varga J, Jimenez SA (1986) Stimulation of normal human fibroblast collagen production and processing by transforming growth factor-beta. Biochem Biophys Res Commun 138:974–980
Ruiz-Ortega M, Rodríguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J (2007) TGF-β signaling in vascular fibrosis. Cardiovasc Res 74:196–206
Allen JL, Cooke ME, Alliston T (2012) ECM stiffness primes the TGFβ pathway to promote chondrocyte differentiation. Mol Biol Cell 23:3731–3742
Thapa N, Lee BH, Kim IS (2007) TGFBIp/betaig-h3 protein: a versatile matrix molecule induced by TGF-beta. Int J Biochem Cell Biol 39:2183–2194
Munier FL, Korvatska E, Djemai A, Le Paslier D, Zografos L, Pescia G, Schorderet DF (1997) Kerato-epithelin mutations in four 5q31-linked corneal dystrophies. Nat Genet 15:247–251
Kim JE, Han MS, Bae YC, Kim HK, Kim TI, Kim EK, Kim IS (2007) Anterior segment dysgenesis after overexpression of transforming growth factor-beta-induced gene, beta igh3, in the mouse eye. Mol Vis 13:1942–1952
Billings PC, Whitbeck JC, Adams CS, Abrams WR, Cohen AJ, Engelsberg BN, Howard PS, Rosenbloom J (2002) The transforming growth factor-beta-inducible matrix protein (beta)ig-h3 interacts with fibronectin. J Biol Chem 277:28003–28009
Hanssen E, Reinboth B, Gibson MA (2003) Covalent and non-covalent interactions of betaig-h3 with collagen VI. Beta ig-h3 is covalently attached to the amino-terminal region of collagen VI in tissue microfibrils. J Biol Chem 278:24334–24341
Reinboth B, Thomas J, Hanssen E, Gibson MA (2006) Beta ig-h3 interacts directly with biglycan and decorin, promotes collagen VI aggregation, and participates in ternary complexing with these macromolecules. J Biol Chem 281:7816–7824
Zhang Y, Wen G, Shao G, Wang C, Lin C, Fang H, Balajee AS, Bhagat G, Hei TK, Zhao Y (2009) TGFBI deficiency predisposes mice to spontaneous tumor development. Cancer Res 69:37–44
Yu H, Wergedal JE, Zhao Y, Mohan S (2012) Targeted disruption of TGFBI in mice reveals its role in regulating bone mass and bone size through periosteal bone formation. Calcif Tissue Int 91:81–87
McDonnell S, Morgan M, Lynch C (1999) Role of matrix metalloproteinases in normal and disease processes. Biochem Soc Trans 27:734–740
Vincenti MP, Coon CI, Mengshol JA, Yocum S, Mitchell P, Brinckerhoff CE (1998) Cloning of the gene for interstitial collagenase-3 (matrix metalloproteinase-13) from rabbit synovial fibroblasts: differential expression with collagenase-1 (matrix metalloproteinase-1). Biochem J 331:341–346
Little C, Barai A, Burkhardt D, Smith S, Fosang A, Werb Z, Shah M, Thompson E (2009) Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum 60:3723–3733
Neuhold LA, Killar L, Zhao W, Sung ML, Warner L, Kulik J, Turner J, Wu W, Billinghurst C, Meijers T, Poole AR, Babij P, DeGennaro LJ (2001) Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest 107:35–44
Acknowledgments
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI13C1874).
Conflict of Interest
Jung-Mi Lee, Eun-Hye Lee, In-San Kim and Jung-Eun Kim state that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All procedures of animal experiments were approved by the Institutional Animal Care and Use Committee of Kyungpook National University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, JM., Lee, EH., Kim, IS. et al. Tgfbi Deficiency Leads to a Reduction in Skeletal Size and Degradation of the Bone Matrix. Calcif Tissue Int 96, 56–64 (2015). https://doi.org/10.1007/s00223-014-9938-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-014-9938-4