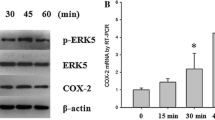
Abstract
Mechanical loading induces positive changes in the skeleton due to direct effects on bone cells, which may include regulation of transcription factors that support osteoblast differentiation and function. Flow effects on osteoblast transcription factors have generally been evaluated after short exposures. In this work, we assayed flow effects on osteogenic genes at early and late time points in a preosteoblast (CIMC-4) cell line and evaluated both steady and oscillatory flows. Four hours of steady unidirectional flow decreased the level of RANKL mRNA 53 ± 7% below that of nonflowed cells, but increases in Runx2 and osterix mRNA (44 ± 22% and 129 ± 12%, respectively) were significant only after 12–19 h of continuous flow. Late flow effects on RANKL and osterix were also induced by an intermittent flow–rest protocol (four cycles of 1 h on/1 h off + overnight rest). Four hours of oscillatory flow decreased RANKL mRNA at this early time point (63 ± 2%) but did not alter either osterix or Runx2. When oscillatory flow was delivered using the intermittent flow–rest protocol, Runx2 and osterix mRNA increased significantly (85 ± 19% and 161 ± 22%, respectively). Both β-catenin and ERK1/2, known to be involved in RANKL regulation, were rapidly activated by steady flow. Inhibition of flow-activated ERK1/2 prevented the increase in osterix mRNA but not Runx2; Runx2 phosphorylation was increased by flow, an effect which likely contributes to osterix induction. This work shows that both steady and oscillatory fluid flows can support enhancement of an osteogenic phenotype.





Similar content being viewed by others
References
Haapasalo H, Kontulainen S, Sievanen H, Kannus P, Jarvinen M, Vuori I (2000) Exercise-induced bone gain is due to enlargement in bone size without a change in volumetric bone density: a peripheral quantitative computed tomography study of the upper arms of male tennis players. Bone 27:351–357
MacKelvie KJ, Khan KM, Petit MA, Janssen PA, McKay HA (2003) A school-based exercise intervention elicits substantial bone health benefits: a 2-year randomized controlled trial in girls. Pediatrics 112:e447
Smith SM, Wastney ME, Morukov BV, Larina IM, Nyquist LE, Abrams SA, Taran EN, Shih CY, Nillen JL, Davis-Street JE, Rice BL, Lane HW (1999) Calcium metabolism before, during, and after a 3-mo spaceflight: kinetic and biochemical changes. Am J Physiol Regul Integr Comp Physiol 277:R1–R10
Uebelhart D, Demiaux-Domenech B, Roth M, Chantraine A (1995) Bone metabolism in spinal cord injured individuals and in others who have prolonged immobilisation. A review. Paraplegia 33:669–673
Whedon G (1984) Disuse osteoporosis: physiologic aspects. Calcif Tissue Int 36:S146–S150
Rubin C, Lanyon L (1984) Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am 66:397–402
Rubin CT, McLeod KJ (1994) Promotion of bony ingrowth by frequency-specific, low-amplitude mechanical strain. Clin Orthop Relat Res 298:165–174
Qin YX, Rubin CT, McLeod KJ (1998) Nonlinear dependence of loading intensity and cycle number in the maintenance of bone mass and morphology. J Orthop Res 16:482–489
Rubin J, Murphy T, Nanes MS, Fan X (2000) Mechanical strain inhibits expression of osteoclast differentiation factor by murine stromal cells. Am J Physiol Cell Physiol 278:C1126–C1132
Li YJ, Batra NN, You L, Meier SC, Coe IA, Yellowley CE, Jacobs CR (2004) Oscillatory fluid flow affects human marrow stromal cell proliferation and differentiation. J Orthop Res 22:1283–1289
Rubin J, Rubin C, Jacobs CR (2006) Molecular pathways mediating mechanical signaling in bone. Gene 367:1–16
You J, Reilly GC, Zhen X, Yellowley CE, Chen Q, Donahue HJ, Jacobs CR (2001) Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in MC3T3–E1 osteoblasts. J Biol Chem 276:13365–13371
Wu CC, Li YS, Haga JH, Wang N, Lian IY, Su FC, Usami S, Chien S (2006) Roles of MAP kinases in the regulation of bone matrix gene expressions in human osteoblasts by oscillatory fluid flow. J Cell Biochem 98:632–641
Harter L, Hruska K, Duncan R (1995) Human osteoblast-like cells respond to mechanical strain with increased bone matrix protein production independent of hormonal regulation. Endocrinology 136:528–535
Arnsdorf EJ, Tummala P, Kwon RY, Jacobs CR (2009) Mechanically induced osteogenic differentiation—the role of RhoA, ROCKII and cytoskeletal dynamics. J Cell Sci 122:546–553
Fan X, Rahnert JA, Murphy TC, Nanes MS, Greenfield EM, Rubin J (2006) Response to mechanical strain in an immortalized pre-osteoblast cell is dependent on ERK1/2. J Cell Physiol 207:454–460
Kim CH, You L, Yellowley CE, Jacobs CR (2006) Oscillatory fluid flow-induced shear stress decreases osteoclastogenesis through RANKL and OPG signaling. Bone 39:1043–1047
Jacobs CR, Yellowley CE, Davis BR, Zhou Z, Cimbala JM, Donahue HJ (1998) Differential effect of steady versus oscillating flow on bone cells. J Biomech 31:969–976
Ponik SM, Triplett JW, Pavalko FM (2007) Osteoblasts and osteocytes respond differently to oscillatory and unidirectional fluid flow profiles. J Cell Biochem 100:794–807
Batra NN, Li YJ, Yellowley CE, You L, Malone AM, Kim CH, Jacobs CR (2005) Effects of short-term recovery periods on fluid-induced signaling in osteoblastic cells. J Biomech 38:1909–1917
Ragab AA, Nalepka JL, Bi Y, Greenfield EM (2002) Cytokines synergistically induce osteoclast differentiation: support by immortalized or normal calvarial cells. Am J Physiol Cell Physiol 283:C679–C687
Case N, Ma M, Sen B, Xie Z, Gross TS, Rubin J (2008) Beta-catenin levels influence rapid mechanical responses in osteoblasts. J Biol Chem 283:29196–29205
Jo H, Song H, Mowbray A (2006) Role of NADPH oxidases in disturbed flow- and BMP4-induced inflammation and atherosclerosis. Antioxid Redox Signal 8:1609–1619
Sen B, Xie Z, Case N, Ma M, Rubin C, Rubin J (2008) Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology 149:6065–6075
van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H (2002) Wnt signaling controls the phosphorylation status of beta-catenin. J Biol Chem 277:17901–17905
Jessop HL, Rawlinson SC, Pitsillides AA, Lanyon LE (2002) Mechanical strain and fluid movement both activate extracellular regulated kinase (ERK) in osteoblast-like cells but via different signaling pathways. Bone 31:186–194
Yang CM, Chien CS, Yao CC, Hsiao LD, Huang YC, Wu CB (2004) Mechanical strain induces collagenase-3 (MMP-13) expression in MC3T3–E1 osteoblastic cells. J Biol Chem 279:22158–22165
Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B (2002) The novel zinc finger–containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108:17–29
Ge C, Xiao G, Jiang D, Yang Q, Hatch NE, Roca H, Franceschi RT (2009) Identification and functional characterization of ERK/MAPK phosphorylation sites in the Runx2 transcription factor. J Biol Chem 284:32533–32543
Chen NX, Ryder KD, Pavalko FM, Turner CH, Burr DB, Qiu J, Duncan RL (2000) Ca2+ regulates fluid shear-induced cytoskeletal reorganization and gene expression in osteoblasts. Am J Physiol Cell Physiol 278:C989–C997
Spencer GJ, Utting JC, Etheridge SL, Arnett TR, Genever PG (2006) Wnt signalling in osteoblasts regulates expression of the receptor activator of NFkappaB ligand and inhibits osteoclastogenesis in vitro. J Cell Sci 119:1283–1296
Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS, Javed A, van Wijnen AJ, Stein JL, Stein GS, Lian JB (2005) Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem 280:33132–33140
Knothe Tate ML (2003) “Whither flows the fluid in bone?” An osteocyte’s perspective. J Biomech 36:1409–1424
Atkins GJ, Kostakis P, Pan B, Farrugia A, Gronthos S, Evdokiou A, Harrison K, Findlay DM, Zannettino AC (2003) RANKL expression is related to the differentiation state of human osteoblasts. J Bone Miner Res 18:1088–1098
Go YM, Boo YC, Park H, Maland MC, Patel R, Pritchard KA Jr, Fujio Y, Walsh K, Darley-Usmar V, Jo H (2001) Protein kinase B/Akt activates c-Jun NH2-terminal kinase by increasing NO production in response to shear stress. J Appl Physiol 91:1574–1581
Traub O, Berk BC (1998) Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol 18:677–685
Srinivasan S, Ausk BJ, Poliachik SL, Warner SE, Richardson TS, Gross TS (2007) Rest-inserted + loading rapidly amplifies the response of bone to small increases in strain and load cycles. J Appl Physiol 102:1945–1952
Robling AG, Burr DB, Turner CH (2000) Partitioning a daily mechanical stimulus into discrete loading bouts improves the osteogenic response to loading. J Bone Miner Res 15:1596–1602
Weyts FA, Li YS, van Leeuwen J, Weinans H, Chien S (2002) ERK activation and alphavbeta3 integrin signaling through Shc recruitment in response to mechanical stimulation in human osteoblasts. J Cell Biochem 87:85–92
Jiang GL, White CR, Stevens HY, Frangos JA (2002) Temporal gradients in shear stimulate osteoblastic proliferation via ERK1/2 and retinoblastoma protein. Am J Physiol Endocrinol Metab 283:E383–E389
Arnsdorf EJ, Tummala P, Jacobs CR (2009) Non-canonical Wnt signaling and N-cadherin related beta-catenin signaling play a role in mechanically induced osteogenic cell fate. PLoS One 4:e5388
Norvell SM, Alvarez M, Bidwell JP, Pavalko FM (2004) Fluid shear stress induces beta-catenin signaling in osteoblasts. Calcif Tissue Int 75:396–404
Sen B, Styner M, Xie Z, Case N, Rubin CT, Rubin J (2009) Mechanical loading regulates NFATC1 and β-catenin signaling through a GSK3β control node. J Biol Chem 284:34607–34617
Smalt R, Mitchell F, Howard R, Chambers T (1997) Induction of NO and prostaglandin E2 in osteoblasts by wall-shear stress but not mechanical strain. Am J Physiol Endocrinol Metab 273:E751–E758
McGarry JG, Klein-Nulend J, Mullender MG, Prendergast PJ (2005) A comparison of strain and fluid shear stress in stimulating bone cell responses—a computational and experimental study. FASEB J 19:482–484
You J, Yellowley CE, Donahue HJ, Zhang Y, Chen Q, Jacobs CR (2000) Substrate deformation levels associated with routine physical activity are less stimulatory to bone cells relative to loading-induced oscillatory fluid flow. J Biomech Eng 122:387–393
Acknowledgement
This work was supported by National Institutes of Health grants AR42360 and AR52014 (to J.R.) and AR045989 (to C. R. J.).
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have stated that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Case, N., Sen, B., Thomas, J.A. et al. Steady and Oscillatory Fluid Flows Produce a Similar Osteogenic Phenotype. Calcif Tissue Int 88, 189–197 (2011). https://doi.org/10.1007/s00223-010-9448-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-010-9448-y