Abstract
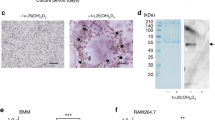
The serum protein prothrombin (PT) is proteolytically converted to thrombin during the coagulation cascade by the cell-associated prothrombinase complex. In vitro, RANKL-differentiated osteoclasts express tissue factor and coagulation factor Xa, which convert PT to thrombin (Karlstrom et al. Biochem Biophys Res Commun 394:593–599, 2010). The present study investigated the localization of PT in bone as well as the expression of PT mRNA in bone and osteoclasts. Herein, immunoblot analysis detected PT and smaller proteolytically cleaved fragments with sizes consistent with the action of prothrombinase in a protein fraction extracted with guanidine-HCl EDTA from mouse tibia. Light microscopic and ultrastructural immunohistochemistry demonstrated the presence of PT in the newly formed bone matrix of the metaphysis. Furthermore, fluorescent immunohistochemistry on metaphyseal trabecular bone showed that PT colocalized with MMP-9-expressing subepiphyseal osteoclasts, whereas cathepsin K-expressing osteoclasts were closely associated with PT of the bone matrix. RT-qPCR analysis revealed that PT mRNA was detected in tibia. Expression of PT mRNA in the tibia was 0.2% of the level in the liver. In addition, PT mRNA expression was increased by RANKL-induced differentiation of bone marrow macrophages to osteoclasts. The results demonstrate that PT is synthesized and proteolytically processed in bone. Furthermore, PT is present mainly in the newly formed bone matrix of the metaphyseal trabecular bone compartment in close association to osteoclasts. In addition, MMP-9-positive osteoclasts contain PT, and PT expression is increased during osteoclastogenesis.






Similar content being viewed by others
References
Mann KG (1976) Prothrombin. Methods Enzymol 45:123–156
Barnhart MI (1960) Cellular site for prothrombin synthesis. Am J Physiol 199:360–366
Jamison CS, Degen SJ (1991) Prenatal and postnatal expression of mRNA coding for rat prothrombin. Biochim Biophys Acta 1088:208–216
Norris LA (2003) Blood coagulation. Best Pract Res Clin Obstet Gynaecol 17:369–383
Lecrone V, Li W, Devoll RE, Logothetis C, Farach-Carson MC (2000) Calcium signals in prostate cancer cells: specific activation by bone-matrix proteins. Cell Calcium 27:35–42
Zoubine MN, Ma JY, Smirnova IV, Citron BA, Festoff BW (1996) A molecular mechanism for synapse elimination: novel inhibition of locally generated thrombin delays synapse loss in neonatal mouse muscle. Dev Biol 179:447–457
Glazner GW, Yadav K, Fitzgerald S, Coven E, Brenneman DE, Nelson PG (1997) Cholinergic stimulation increases thrombin activity and gene expression in cultured mouse muscle. Brain Res Dev Brain Res 99:148–154
Flynn PD, Byrne CD, Baglin TP, Weissberg PL, Bennett MR (1997) Thrombin generation by apoptotic vascular smooth muscle cells. Blood 89:4378–4384
Festoff BW, Smirnova IV, Ma J, Citron BA (1996) Thrombin, its receptor and protease nexin I, its potent serpin, in the nervous system. Semin Thromb Hemost 22:267–271
Suzuki K, Tanaka T, Miyazawa K, Nakajima C, Moriyama M, Suga K, Murai M, Yano J (1999) Gene expression of prothrombin in human and rat kidneys: basic and clinical approach. J Am Soc Nephrol 10(Suppl 14):S408–S411
Trudel G, Uhthoff HK, Laneuville O (2005) Prothrombin gene expression in articular cartilage with a putative role in cartilage degeneration secondary to joint immobility. J Rheumatol 32:1547–1555
Osterud B, Lindahl U, Seljelid R (1980) Macrophages produce blood coagulation factors. FEBS Lett 120:41–43
Shim K, Zhu H, Westfield LA, Sadler JE (2004) A recombinant murine meizothrombin precursor, prothrombin R157A/R268A, inhibits thrombosis in a model of acute carotid artery injury. Blood 104:415–419
Butenas S, Mann KG (2002) Blood coagulation. Biochemistry (Mosc) 67:3–12
Ahmad SS, London FS, Walsh PN (2003) The assembly of the factor X-activating complex on activated human platelets. J Thromb Haemost 1:48–59
Krishnaswamy S, Mann KG, Nesheim ME (1986) The prothrombinase-catalyzed activation of prothrombin proceeds through the intermediate meizothrombin in an ordered, sequential reaction. J Biol Chem 261:8977–8984
Mann KG, Nesheim ME, Church WR, Haley P, Krishnaswamy S (1990) Surface-dependent reactions of the vitamin K-dependent enzyme complexes. Blood 76:1–16
Pagel CN, Sivagurunathan S, Loh LH, Tudor EM, Pike RN, Mackie EJ (2006) Functional responses of bone cells to thrombin. Biol Chem 387:1037–1041
Gabazza EC, Taguchi O, Kamada H, Hayashi T, Adachi Y, Suzuki K (2004) Progress in the understanding of protease-activated receptors. Int J Hematol 79:117–122
Pagel CN, de Niese MR, Abraham LA, Chinni C, Song SJ, Pike RN, Mackie EJ (2003) Inhibition of osteoblast apoptosis by thrombin. Bone 33:733–743
Jenkins AL, Bootman MD, Taylor CW, Mackie EJ, Stone SR (1993) Characterization of the receptor responsible for thrombin-induced intracellular calcium responses in osteoblast-like cells. J Biol Chem 268:21432–21437
Abraham LA, Jenkins AL, Stone SR, Mackie EJ (1998) Expression of the thrombin receptor in developing bone and associated tissues. J Bone Miner Res 13:818–827
Frost A, Jonsson KB, Ridefelt P, Nilsson O, Ljunghall S, Ljunggren O (1999) Thrombin, but not bradykinin, stimulates proliferation in isolated human osteoblasts, via a mechanism not dependent on endogenous prostaglandin formation. Acta Orthop Scand 70:497–503
Song SJ, Pagel CN, Campbell TM, Pike RN, Mackie EJ (2005) The role of protease-activated receptor-1 in bone healing. Am J Pathol 166:857–868
Ljunggren O, Johansson H, Ljunghall S, Lerner UH (1991) Thrombin increases cytoplasmic Ca2+ and stimulates formation of prostaglandin E2 in the osteoblastic cell line MC3T3-El. Bone Miner 12:81–90
Kozawa O, Tokuda H, Kaida T, Matsuno H, Uematsu T (1997) Thrombin regulates interleukin-6 synthesis through phosphatidylcholine hydrolysis by phospholipase D in osteoblasts. Arch Biochem Biophys 345:10–15
Shinohara M, Takayanagi H (2007) Novel osteoclast signaling mechanisms. Curr Osteoporos Rep 5:67–72
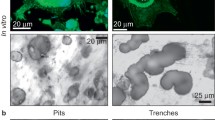
Hu Y, Ek-Rylander B, Karlstrom E, Wendel M, Andersson G (2008) Osteoclast size heterogeneity in rat long bones is associated with differences in adhesive ligand specificity. Exp Cell Res 314:638–650
Karlstrom E, Ek-Rylander B, Wendel M, Andersson G (2010) RANKL induces components of the extrinsic coagulation pathway in osteoclasts. Biochem Biophys Res Commun 394:593–599
Hultenby K, Reinholt FP, Oldberg A, Heinegard D (1991) Ultrastructural immunolocalization of osteopontin in metaphyseal and cortical bone. Matrix 11:206–213
Van Noorden S (1986) Immunocytochemistry, modern methods and applications. Wright, Bristol
Zenger S, Hollberg K, Ljusberg J, Norgard M, Ek-Rylander B, Kiviranta R, Andersson G (2007) Proteolytic processing and polarized secretion of tartrate-resistant acid phosphatase is altered in a subpopulation of metaphyseal osteoclasts in cathepsin K-deficient mice. Bone 41:820–832
Takeshita S, Kaji K, Kudo A (2000) Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J Bone Miner Res 15:1477–1488
Fischer BE, Schlokat U, Mitterer A, Grillberger L, Reiter M, Mundt W, Dorner F, Eibl J (1996) Differentiation between proteolytic activation and autocatalytic conversion of human prothrombin. Activation of recombinant human prothrombin and recombinant D419N-prothrombin by snake venoms from Echis carinatus and Oxyuranus scutellatus. Protein Eng 9:921–926
Jemtland R, Lee K, Segre GV (1998) Heterogeneity among cells that express osteoclast-associated genes in developing bone. Endocrinology 139:340–349
Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z (1998) MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 93:411–422
Saftig P, Hunziker E, Wehmeyer O, Jones S, Boyde A, Rommerskirch W, Moritz JD, Schu P, von Figura K (1998) Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc Natl Acad Sci USA 95:13453–13458
Tezuka K, Tezuka Y, Maejima A, Sato T, Nemoto K, Kamioka H, Hakeda Y, Kumegawa M (1994) Molecular cloning of a possible cysteine proteinase predominantly expressed in osteoclasts. J Biol Chem 269:1106–1109
Everts V, de Vries TJ, Helfrich MH (2009) Osteoclast heterogeneity: lessons from osteopetrosis and inflammatory conditions. Biochim Biophys Acta 1792:757–765
Nordahl J, Andersson G, Reinholt FP (1998) Chondroclasts and osteoclasts in bones of young rats: comparison of ultrastructural and functional features. Calcif Tissue Int 63:401–408
Robey PG (2002) Bone matrix proteoglycans and glycoproteins. In: Bilezikian JP, Raisz LA, Rodan GA (eds) Principles of bone biology. Academic Press, San Diego, CA, pp 225–237
Lian SG, Canalis E, Robey PG, Boskey AL (1999) Bone formation: osteoblast lineage cells, growth factors, matrix proteins and the mineralization process. Lippincott, Williams & Williams, Philadelphia
van den Bos T, Speijer D, Bank RA, Bromme D, Everts V (2008) Differences in matrix composition between calvaria and long bone in mice suggest differences in biomechanical properties and resorption: special emphasis on collagen. Bone 43:459–468
Senger DR, Perruzzi CA, Papadopoulos-Sergiou A, Van de Water L (1994) Adhesive properties of osteopontin: regulation by a naturally occurring thrombin-cleavage in close proximity to the GRGDS cell-binding domain. Mol Biol Cell 5:565–574
Hasegawa M, Nakoshi Y, Iino T, Sudo A, Segawa T, Maeda M, Yoshida T, Uchida A (2009) Thrombin-cleaved osteopontin in synovial fluid of subjects with rheumatoid arthritis. J Rheumatol 36:240–245
Yokasaki Y, Sheppard D (2000) Mapping of the cryptic integrin-binding site in osteopontin suggests a new mechanism by which thrombin can regulate inflammation and tissue repair. Trends Cardiovasc Med 10:155–159
Yokosaki Y, Matsuura N, Sasaki T, Murakami I, Schneider H, Higashiyama S, Saitoh Y, Yamakido M, Taooka Y, Sheppard D (1999) The integrin alpha9beta1 binds to a novel recognition sequence (SVVYGLR) in the thrombin-cleaved amino-terminal fragment of osteopontin. J Biol Chem 274:36328–36334
Rao H, Lu G, Kajiya H, Garcia-Palacios V, Kurihara N, Anderson J, Patrene K, Sheppard D, Blair HC, Windle JJ, Choi SJ, Roodman GD (2006) Alpha9beta1: a novel osteoclast integrin that regulates osteoclast formation and function. J Bone Miner Res 21:1657–1665
Yamamoto N, Sakai F, Kon S, Morimoto J, Kimura C, Yamazaki H, Okazaki I, Seki N, Fujii T, Uede T (2003) Essential role of the cryptic epitope SLAYGLR within osteopontin in a murine model of rheumatoid arthritis. J Clin Invest 112:181–188
Acknowledgements
This work was supported by grants from the Swedish Research Council and the Stockholm County Council.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have stated that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Karlström, E., Norgård, M., Hultenby, K. et al. Localization and Expression of Prothrombin in Rodent Osteoclasts and Long Bones. Calcif Tissue Int 88, 179–188 (2011). https://doi.org/10.1007/s00223-010-9443-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-010-9443-3