Abstract
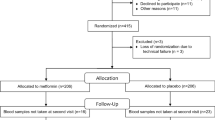
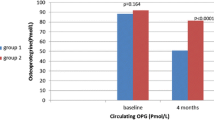
In previous studies, with up to 16 weeks of exposure to rosiglitazone or pioglitazone, circulating markers of bone formation [procollagen I N-terminal propeptide (P1NP), osteocalcin, and bone-specific alkaline phosphatase] decreased but no change in bone resorption markers was found. We examined the effect of rosiglitazone on bone resorption and formation markers when used for 24 weeks. This post-hoc analysis of a double-blind, placebo-controlled, randomized trial evaluated the effects of 6 months of rosiglitazone use versus placebo on circulating markers of bone turnover in 111 patients with type 2 diabetes and cardiovascular disease or additional cardiac risk factors. The principal end points for analysis were changes in bone formation and resorption markers, measured by P1NP and carboxy-terminal cross-links (CTX), respectively. There were 111 subjects who completed the study and had baseline and 6-month data; mean age was 56, including 41% women and 67% nonwhite (50 black, 18 Hispanic, and six other), and subjects were evenly distributed between placebo and rosiglitazone groups. Women treated with rosiglitazone had higher CTX levels (0.43 ng/mL) than those who received placebo (0.23 ng/mL) (P = 0.007), with no significant differences in P1NP or OPG. Overall, in stratified analyses of men and in stratified analyses among different ethnicities, there were no statistically significant differences observed in CTX, P1NP, OPG, PTH, or 25-OHD between the treatment groups. Women taking rosiglitazone had higher circulating markers of bone resorption, which is contrary to prior studies of shorter duration, where the principal observation was a decrease in markers of bone formation.


Similar content being viewed by others
References
Yki-Jarvinen H (2004) Thiazolidinediones. N Engl J Med 351:1106–1118
Avandia (2007) Highlights of prescribing information [package insert]. Glaxo Smith Kline, pp 1–42
Actos (2007) Prescribing information. Takeda Pharmaceutical Company, pp 1–4
Ali AA, Weinstein RS, Stewart SA, Parfitt AM, Manolagas SC, Jilka RL (2005) Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology 146:1226–1235
Lazarenko OP, Rzonca SO, Hogue WR, Swain FL, Suva LJ, Lecka-Czernik B (2007) Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone. Endocrinology 148:2669–2680
Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka-Czernik B (2004) Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology 145:401–406
Soroceanu MA, Miao D, Bai XY, Su H, Goltzman D, Karaplis AC (2004) Rosiglitazone impacts negatively on bone by promoting osteoblast/osteocyte apoptosis. J Endocrinol 183:203–216
Grey A, Bolland M, Gamble G, Wattie D, Horne A, Davidson J, Reid IR (2007) The peroxisome proliferator-activated receptor-gamma agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab 92:1305–1310
Schwartz AV, Sellmeyer DE (2007) Thiazolidinedione therapy gets complicated: is bone loss the price of improved insulin resistance? Diabetes Care 30:1670–1671
Yaturu S, Bryant B, Jain SK (2007) Thiazolidinedione treatment decreases bone mineral density in type 2 diabetic men. Diabetes Care 30:1574–1576
Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B, Viberti G (2006) Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355:2427–2443
Meymeh RH, Wooltorton E (2007) Diabetes drug pioglitazone (Actos): risk of fracture. CMAJ 177:723–724
Nissen SE, Nicholls SJ, Wolski K, Nesto R, Kupfer S, Perez A, Jure H, De Larochelliere R, Staniloae CS, Mavromatis K, Saw J, Hu B, Lincoff AM, Tuzcu EM (2008) Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA 299:1561–1573
Meier C, Kraenzlin ME, Bodmer M, Jick SS, Jick H, Meier CR (2008) Use of thiazolidinediones and fracture risk. Arch Intern Med 168:820–825
Hampton T (2007) Diabetes drugs tied to fractures in women. JAMA 297:1645
McGuire DK, See R, Abdullah SM, Snell PG, McGavock JM, Ayers CR, Szczepaniak LS (2009) The effect of rosiglitazone on integrated cardiovascular performance, cardiac structure, function and myocardial triglyceride: trial design and rationale. Diab Vasc Dis Res 6:43–50
Abedin M, Omland T, Ueland T, Khera A, Aukrust P, Murphy SA, Jain T, Gruntmanis U, McGuire DK, de Lemos JA (2007) Relation of osteoprotegerin to coronary calcium and aortic plaque (from the Dallas Heart Study). Am J Cardiol 99:513–518
Reid IR, Davidson JS, Wattie D, Wu F, Lucas J, Gamble GD, Rutland MD, Cundy T (2004) Comparative responses of bone turnover markers to bisphosphonate therapy in Paget’s disease of bone. Bone 35:224–230
Reid IR, Lucas J, Wattie D, Horne A, Bolland M, Gamble GD, Davidson JS, Grey AB (2005) Effects of a beta-blocker on bone turnover in normal postmenopausal women: a randomized controlled trial. J Clin Endocrinol Metab 90:5212–5216
ALPCO (2007) Intact PTH (1–84) EIA, pp 1–13. http://www.alpco.com/products/PTH_1-84_ELISA.asp
Berberoglu Z, Gursoy A, Bayraktar N, Yazici AC, Bascil Tutuncu N, Guvener Demirag N (2007) Rosiglitazone decreases serum bone-specific alkaline phosphatase activity in postmenopausal diabetic women. J Clin Endocrinol Metab 92:3523–3530
Glintborg D, Andersen M, Hagen C, Heickendorff L, Hermann AP (2008) Association of pioglitazone treatment with decreased bone mineral density in obese premenopausal patients with polycystic ovary syndrome: a randomized, placebo-controlled trial. J Clin Endocrinol Metab 93:1696–1701
Benvenuti S, Cellai I, Luciani P, Deledda C, Baglioni S, Giuliani C, Saccardi R, Mazzanti B, Dal Pozzo S, Mannucci E, Peri A, Serio M (2007) Rosiglitazone stimulates adipogenesis and decreases osteoblastogenesis in human mesenchymal stem cells. J Endocrinol Invest 30:RC26–RC30
Wan Y, Chong LW, Evans RM (2007) PPARgamma regulates osteoclastogenesis in mice. Nat Med 13(12):1496–1503
Sottile V, Seuwen K, Kneissel M (2004) Enhanced marrow adipogenesis and bone resorption in estrogen-deprived rats treated with the PPARgamma agonist BRL49653 (rosiglitazone). Calcif Tissue Int 75:329–337
Aleo MD, Lundeen GR, Blackwell DK, Smith WM, Coleman GL, Stadnicki SW, Kluwe WM (2003) Mechanism and implications of brown adipose tissue proliferation in rats and monkeys treated with the thiazolidinedione darglitazone, a potent peroxisome proliferator-activated receptor-gamma agonist. J Pharmacol Exp Ther 305:1173–1182
Li M, Pan LC, Simmons HA, Li Y, Healy DR, Robinson BS, Ke HZ, Brown TA (2006) Surface-specific effects of a PPARgamma agonist, darglitazone, on bone in mice. Bone 39:796–806
Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, Garnero P, Bouxsein ML, Bilezikian JP, Rosen CJ (2003) The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 349:1207–1215
Borba VZ, Paz-Filho G, Kulak CA, Seibel MJ, Bilezikian JP (2007) Bone turnover 18 months after a single intravenous dose of zoledronic acid. Int J Clin Pract 61:1058–1062
Kulak CA, Borba VZ, Silvado CE, Paola L, Seibel MJ, Bilezikian JP, Boguszewski CL (2007) Bone density and bone turnover markers in patients with epilepsy on chronic antiepileptic drug therapy. Arq Bras Endocrinol Metabol 51:466–471
Tohme JF, Seibel MJ, Silverberg SJ, Robins SP, Bilezikian JP (1991) Biochemical markers of bone metabolism. Z Rheumatol 50:133–141
Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, Petruschke RA, Chen E, de Papp AE (2005) Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab 90:3215–3224
Levis S, Gomez A, Jimenez C, Veras L, Ma F, Lai S, Hollis B, Roos BA (2005) Vitamin D deficiency and seasonal variation in an adult South Florida population. J Clin Endocrinol Metab 90:1557–1562
Hannan MT, Litman HJ, Araujo AB, McLennan CE, McLean RR, McKinlay JB, Chen TC, Holick MF (2008) Serum 25-hydroxyvitamin D and bone mineral density in a racially and ethnically diverse group of men. J Clin Endocrinol Metab 93:40–46
Holick MF (2006) High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 81:353–373
Loke YK, Singh S, Furberg CD (2009) Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. CMAJ 180:32–39
Acknowledgement
This work was supported by grants from GlaxoSmithKline, Biosite, and the Donald W. Reynolds Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ugis Gruntmanis, Steve Fordan contributed equally to this work.
U. G. has received research support from Procter & Gamble, Novartis, GlaxoSmithKline, and Amgen. D. K. M. was previously on speakers’ bureaus for Takeda and Pfizer and is a consultant with Novo Nordisk, Johnson and Johnson, and Tethys Bioscience. All other authors have no conflict of interest.
Rights and permissions
About this article
Cite this article
Gruntmanis, U., Fordan, S., Ghayee, H.K. et al. The Peroxisome Proliferator-Activated Receptor-γ Agonist Rosiglitazone Increases Bone Resorption in Women with Type 2 Diabetes: A Randomized, Controlled Trial. Calcif Tissue Int 86, 343–349 (2010). https://doi.org/10.1007/s00223-010-9352-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-010-9352-5