Abstract
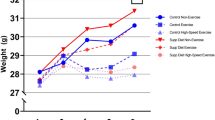
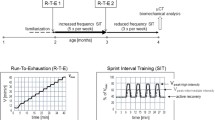
We have previously shown that exercise during growth increases post-yield deformation in C57BL6/129 (B6;129) male tibiae at the expense of reduced pre-yield deformation and structural and tissue strength. Other research in the literature indicates that increased mineral content, cross-sectional geometry and structural strength due to exercise can be maintained or increased after exercise ends for as long as 14 weeks. It was therefore hypothesized that after our exercise protocol ended, effects of exercise on mechanical properties would persist, resulting in increased post-yield behavior and rescued strength versus age-matched control mice. Beginning at 8 weeks of age, exercise consisted of running on a treadmill (30 min/day, 12 m/min, 5° incline) for 21 consecutive days. At the end of running and 2 weeks later, in the cortical bone of the tibial mid-diaphyses of B6;129 male mice, changes due to exercise and latency following exercise were assayed by mechanical tests and analyses of cross-sectional geometry. Exercise increased structural post-yield deformation compared with weight-matched control mice, without changes in bone size or shape, suggesting that exercised-induced changes in pre-existing bone quality were responsible. Over the 2-week latency period, no growth-related changes were noted in control mice, but exercise-induced changes resulted in increased tissue stiffness and strength versus mice sacrificed immediately after exercise ended. Our data indicate that periods of exercise followed by latency can alter strength, stiffness, and ductility of bone independent of changes in size or shape, suggesting that exercise may be a practical way to increase the quality of the bone extracellular matrix.



Similar content being viewed by others
References
Mori T, Okimoto N, Sakai A, Okazaki Y, Nakura N, Notomi T, Nakamura T (2003) Climbing exercise increases bone mass and trabecular bone turnover through transient regulation of marrow osteogenic and osteoclastogenic potentials in mice. J Bone Miner Res 18:2002–2009
Wu J, Wang XX, Higuchi M, Yamada K, Ishimi Y (2004) High bone mass gained by exercise in growing male mice is increased by subsequent reduced exercise. J Appl Physiol 97:806–810
Hoshi A, Watanabe H, Chiba M, Inaba Y (1998) Bone density and mechanical properties in femoral bone of swim loaded aged mice. Biomed Environ Sci 11:243–250
Gordon KR, Levy C, Perl M, Weeks OI (1994) Experimental perturbation of the development of sexual size dimorphism in the mouse skeleton. Growth Dev Aging 58:95–104
Kodama Y, Umemura Y, Nagasawa S, Beamer WG, Donahue LR, Rosen CR, Baylink DJ, Farley JR (2000) Exercise and mechanical loading increase periosteal bone formation and whole bone strength in C57BL/6 J mice but not in C3H/Hej mice. Calcif Tissue Int 66:298–306
Umemura Y, Ishiko T, Yamauchi T, Kurono M, Mashiko S (1997) Five jumps per day increase bone mass and breaking force in rats. J Bone Miner Res 12:1480–1485
Iwamoto J, Yeh JK, Aloia JF (2000) Effect of deconditioning on cortical and cancellous bone growth in the exercise trained young rats. J Bone Miner Res 15:1842–1849
Notomi T, Okimoto N, Okazaki Y, Tanaka Y, Nakamura T, Suzuki M (2001) Effects of tower climbing exercise on bone mass, strength, and turnover in growing rats. J Bone Miner Res 16:166–174
Huang TH, Lin SC, Chang FL, Hsieh SS, Liu SH, Yang RS (2003) Effects of different exercise modes on mineralization, structure, and biomechanical properties of growing bone. J Appl Physiol 95:300–307
Wolff J (1892) Das gesetz der transformation der knochen. August Hirschwald, Berlin
Wallace JM, Rajachar RM, Allen MR, Bloomfield SA, Robey PG, Young MF, Kohn DH (2007) Exercise-induced changes in the cortical bone of growing mice are bone- and gender-specific. Bone 40:1120–1127
Singh R, Umemura Y, Honda A, Nagasawa S (2002) Maintenance of bone mass and mechanical properties after short-term cessation of high impact exercise in rats. Int J Sports Med 23:77–81
Pajamaki I, Kannus P, Vuohelainen T, Sievanen H, Tuukkanen J, Jarvinen M, Jarvinen TL (2003) The bone gain induced by exercise in puberty is not preserved through a virtually life-long deconditioning: a randomized controlled experimental study in male rats. J Bone Miner Res 18:544–552
Tay BK, Le AX, Gould SE, Helms JA (1998) Histochemical and molecular analyses of distraction osteogenesis in a mouse model. J Orthop Res 16:636–642
Carvalho RS, Einhorn TA, Lehmann W, Edgar C, Al-Yamani A, Apazidis A, Pacicca D, Clemens TL, Gerstenfeld LC (2004) The role of angiogenesis in a murine tibial model of distraction osteogenesis. Bone 34:849–861
Isefuku S, Joyner CJ, Simpson AH (2000) A murine model of distraction osteogenesis. Bone 27:661–665
Moore DS, McCabe GP (2003) Introduction to the practice of statistics. W. H. Freeman, New York
Wallace JM, Rajachar RM, Chen XD, Shi S, Allen MR, Bloomfield SA, Les CM, Robey PG, Young MF, Kohn DH (2006) The mechanical phenotype of biglycan-deficient mice is bone- and gender-specific. Bone 39:106–116
Turner CH, Burr DB (1993) Basic biomechanical measurements of bone: a tutorial. Bone 14:595–607
Boskey AL, Wright TM, Blank RD (1999) Collagen and bone strength. J Bone Miner Res 14:330–335
Burstein AH, Zika JM, Heiple KG, Klein L (1975) Contribution of collagen and mineral to the elastic-plastic properties of bone. J Bone Joint Surg Am 57:956–961
Wang X, Shen X, Li X, Agrawal CM (2002) Age-related changes in the collagen network and toughness of bone. Bone 31:1–7
Garnero P, Borel O, Gineyts E, Duboeuf F, Solberg H, Bouxsein ML, Christiansen C, Delmas PD (2006) Extracellular post-translational modifications of collagen are major determinants of biomechanical properties of fetal bovine cortical bone. Bone 38:300–309
Viguet-Carrin S, Garnero P, Delmas PD (2006) The role of collagen in bone strength. Osteoporos Int 17:319–336
Kohn DH, Sahar ND, Wallace JM, Golcuk K, Morris MD (2009) Exercise alters mineral and matrix composition in the absence of adding new bone. Cells Tissues Organs 189:33–37
Currey JD (1988) The effect of porosity and mineral content on the Young’s modulus of elasticity of compact bone. J Biomech 21:131–139
Wallace JM (2007) Investigating the inbred strain-specific response to biglycan-deficiency and exercise: a study in genetically-medicated skeletal adaptation. Ph.D. dissertation. University of Michigan. Available at: http://hdl.handle.net/2027.42/57673
Qing H, Ardeshirpour L, Dusevich V, Dallas M, Wysolmerski JJ, Bonewald LF (2008) Osteocytic perilacunar remodeling as a significant source of calcium during lactation. ASBMR Abstract M110
Carden A, Rajachar RM, Morris MD, Kohn DH (2003) Ultrastructural changes accompanying the mechanical deformation of bone tissue: a Raman imaging study. Calcif Tissue Int 72:166–175
Vaidya S, Karunakaran C, Pande B, Gupta N, Iyer R, Karweer S (1997) Pressure-induced crystalline to amorphous transition in hydroxylapatite. J Mater Sci 32:3213–3217
Gupta HS, Seto J, Wagermaier W, Zaslansky P, Boesecke P, Fratzl P (2006) Cooperative deformation of mineral and collagen in bone at the nanoscale. Proc Natl Acad Sci USA 103:17741–17746
Wilson EE, Awonusi A, Morris MD, Kohn DH, Tecklenburg MM, Beck LW (2005) Highly ordered interstitial water observed in bone by nuclear magnetic resonance. J Bone Miner Res 20:625–634
Wilson EE, Awonusi A, Morris MD, Kohn DH, Tecklenburg MM, Beck LW (2006) Three structural roles for water in bone observed by solid-state NMR. Biophys.J 90:3722–3731
Carter DR, Van Der Meulen MC, Beaupre GS (1996) Mechanical factors in bone growth and development. Bone 18:5S–10S
Rubin C, Xu G, Judex S (2001) The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low-magnitude mechanical stimuli. FASEB J 15:2225–2229
Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K (2004) Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res 19:343–351
Ward K, Alsop C, Caulton J, Rubin C, Adams J, Mughal Z (2004) Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res 19:360–369
Carleton SM, Weber B, McCambridge A, Ferriera J, Brown MB, Phillips CL (2008) Impact of exercise on skeletal muscle and bone in oim mice. Matrix Biol 27:24
Wu J, Wang XX, Takasaki M, Ohta A, Higuchi M, Ishimi Y (2001) Cooperative effects of exercise training and genistein administration on bone mass in ovariectomized mice. J Bone Miner Res 16:1829–1836
Forwood MR, Owan I, Takano Y, Turner CH (1996) Increased bone formation in rat tibiae after a single short period of dynamic loading in vivo. Am J Physiol 270:E419–E423
Rubin CT, Lanyon LE (1984) Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am 66:397–402
Boskey AL (2003) Bone mineral crystal size. Osteoporos Int 14(Suppl 5):16–21
Acknowledgments
This study received funding from DoD/US Army DAMD17-03-1-0556, an NIH IPA Agreement and Regenerative Sciences Training Grant R90-DK071506.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wallace, J.M., Ron, M.S. & Kohn, D.H. Short-Term Exercise in Mice Increases Tibial Post-Yield Mechanical Properties While Two Weeks of Latency Following Exercise Increases Tissue-Level Strength. Calcif Tissue Int 84, 297–304 (2009). https://doi.org/10.1007/s00223-009-9228-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-009-9228-8