Abstract
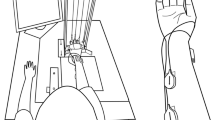
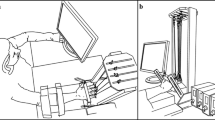
We explored the synergic organization of motor units in extrinsic finger muscles, flexor digitorum superficialis (FDS), and extensor digitorum communis (EDC). Healthy subjects produced accurate cyclical force by pressing with the middle phalanges of one of the three fingers (Index, Middle, and Ring) and all three together. Two wireless sensor arrays were used to record and identify motor unit action potentials in FDS and EDC. Stable motor unit groups were identified within each muscle and across both muscles. Analysis of motor units combined over the two muscles showed one of the first two motor unit groups with consistently opposite signs of the loading factors for the FDS and EDC motor units, and the other group with consistently same signs of the loading factors for the two muscles. We interpret the two motor unit groups as reflections of the reciprocal and co-activation commands within the theory of control with spatial referent coordinates. Force changes within the cycle were primarily associated with the modulation of the co-activation motor unit group. Analysis of inter-cycle variance within the spaces of motor unit groups defined for FDS and EDC separately showed force-stabilizing synergies across both single-finger and three-finger tasks. In contrast, analysis within the motor unit groups defined across both muscles failed to show force-stabilizing synergies. We interpret these results as a reflection of the trade-off across levels within a hierarchical control system.











Similar content being viewed by others
Availability of data and material
The original data are available from the corresponding author at a reasonable request.
Code availability
The original codes in Matlab are available from the corresponding author at a reasonable request.
References
Ambike S, Zhou T, Zatsiorsky VM, Latash ML (2015) Moving a hand-held object: reconstruction of referent coordinate and apparent stiffness trajectories. Neuroscience 298:336–356
Ambike S, Mattos D, Zatsiorsky VM, Latash ML (2016) Synergies in the space of control variables within the equilibrium-point hypothesis. Neuroscience 315:150–161
Babinski F (1899) De l’asynergie cerebelleuse. Revue Neurologique 7:806–816
Bernstein NA (1947) On the construction of movements (in Russian). In: Latash ML (ed) Bernstein’s construction of movements (2020). Medgiz, Moscow, pp 1–220
Butler TJ, Kilbreath SL, Gorman RB, Gandevia SC (2005) Selective recruitment of single motor units in human flexor digitorum superficialis muscle during flexion of individual fingers. J Physiol 567:301–309
Corcos DM, Agarwal GC, Flaherty BP, Gottlieb GL (1990) Organizing principles for single-joint movements: IV. Implications for isometric contractions. J Neurophysiol 64:1033–1042
Cuadra C, Bartsch A, Tiemann P, Reschechtko S, Latash ML (2018) Multi-finger synergies and the muscular apparatus of the hand. Exp Brain Res 236:1383–1393
Cuadra C, Wojnicz W, Kozinc Z, Latash ML (2020) Perceptual and motor effects of muscle co-activation in a force production task. Neuroscience 437:34–44
Danna-Dos-Santos A, Slomka K, Zatsiorsky VM, Latash ML (2007) Muscle modes and synergies during voluntary body sway. Exp Brain Res 179:533–550
d’Avella A, Saltiel P, Bizzi E (2003) Combinations of muscle synergies in the construction of a natural motor behavior. Nat Neurosci 6:300–308
De Luca CJ, Chang SS, Roy SH, Kline JC, Nawab SH (2015) Decomposition of surface EMG signals from cyclic dynamic contractions. J Neurophysiol 113:1941–1951
Falaki A, Huang X, Lewis MM, Latash ML (2016) Impaired synergic control of posture in Parkinson’s patients without postural instability. Gait Posture 44:209–215
Feldman AG (1966) Functional tuning of the nervous system with control of movement or maintenance of a steady posturez. II. Controllable parameters of the muscle. Biophysics 11:565–578
Feldman AG (1980) Superposition of motor programs. I. Rhythmic forearm movements in man. Neuroscience 5:81–90
Feldman AG (1981) The composition of central programs subserving horizontal eye movements in man. Biol Cybern 42:107–116
Feldman AG (1986) Once more on the equilibrium-point hypothesis (λ–model) for motor control. J Mot Behav 18:17–54
Feldman AG (2015) Referent control of action and perception: challenging conventional theories in behavioral science. Springer, New York
Feldman AG, Levin MF, Garofolini A, Piscitelli D, Zhang L (2021) Central pattern generator and human locomotion in the context of referent control of motor actions. Clin Neurophysiol 132:2870–2889
Freitas SMSF, de Freitas PB, Lewis MM, Huang X, Latash ML (2019) Quantitative analysis of multi-element synergies stabilizing performance: comparison of three methods with respect to their use in clinical studies. Exp Brain Res 237:453–465
Friedman J, Varadhan SKM, Zatsiorsky VM, Latash ML (2009) The sources of two components of variance: an example of multifinger cyclic force production tasks at different frequencies. Exp Brain Res 196:263–277
Frysinger RC, Bourbonnais D, Kalaska JF, Smith AM (1984) Cerebellar cortical activity during antagonist cocontraction and reciprocal inhibition of forearm muscles. J Neurophysiol 51:32–49
Ghez C, Gordon J (1987) Trajectory control in targeted force impulses. I. Role of opposing muscles. Exp Brain Res 67:225–240
Gorniak S, Zatsiorsky VM, Latash ML (2007) Hierarchies of synergies: an example of the two-hand, multi-finger tasks. Exp Brain Res 179:167–180
Gorniak S, Zatsiorsky VM, Latash ML (2009) Hierarchical control of static prehension: II. multi-digit synergies. Exp Brain Res 194:1–15
Henneman E, Somjen G, Carpenter DO (1965) Excitability and inhibitibility of motoneurones of different sizes. J Neurophysiol 28:599–620
Hirokawa S, Solomonow M, Luo Z, Lu Y, D’Ambrosia R (1991) Muscular co-contraction and control of knee stability. J Electromyogr Kinesiol 1:199–208
Hogan N, Sternad D (2012) Dynamic primitives of motor behavior. Biol Cybern 106:727–739
Hultborn H, Jankowska E, Lindstrom S (1971) Recurrent inhibition from motor axon collaterals of transmission in the Ia inhibitory pathway to motoneurons. J Physiol 215:591–612
Hultborn H, Lindström S, Wigström H (1979) On the function of recurrent inhibition in the spinal cord. Exp Brain Res 37:399–403
Hultborn H, Brownstone RB, Toth TI, Gossard JP (2004) Key mechanisms for setting the input-output gain across the motoneuron pool. Prog Brain Res 143:77–95
Ivanenko YP, Poppele RE, Lacquaniti F (2004) Five basic muscle activation patterns account for muscle activity during human locomotion. J Physiol 556:267–282
Ivanenko YP, Poppele RE, Lacquaniti F (2006) Motor control programs and walking. Neuroscientist 12:339–348
Jeneson JA, Taylor JS, Vigneron DB, Willard TS, Carvajal L, Nelson SJ, Murphy-Boesch J, Brown TR (1990) 1H MR imaging of anatomical compartments within the finger flexor muscles of the human forearm. Magn Reson Med 15:491–496
Krishnamoorthy V, Goodman SR, Latash ML, Zatsiorsky VM (2003) Muscle synergies during shifts of the center of pressure by standing persons: identification of muscle modes. Biol Cybern 89:152–161
Lacquaniti F, Ivanenko YP, Zago P (2012) Development of human locomotion. Curr Opin Neurobiol 22:822–828
Latash ML (1992) Virtual trajectories, joint stiffness, and changes in natural frequency during single-joint oscillatory movements. Neuroscience 49:209–220
Latash ML (2008) Synergy. Oxford University Press, New York
Latash ML (2010) Motor synergies and the equilibrium-point hypothesis. Mot Control 14:294–322
Latash ML (2012) The bliss (not the problem) of motor abundance (not redundancy). Exp Brain Res 217:1–5
Latash ML (2018) Muscle co-activation: definitions, mechanisms, and functions. J Neurophysiol 120:88–104
Latash ML (2019) Physics of biological action and perception. Academic Press, New York
Latash ML (2020a) On primitives in motor control. Mot Control 24:318–346
Latash ML (ed) (2020b) Bernstein’s construction of movements. Routledge, Abingdon
Latash ML (2021) Laws of nature that define biological action and perception. Phys Life Rev 36:47–67
Latash ML, Gottlieb GL (1991) Reconstruction of elbow joint compliant characteristics during fast and slow voluntary movements. Neuroscience 43:697–712
Latash ML, Huang X (2015) Neural control of movement stability: lessons from studies of neurological patients. Neuroscience 301:39–48
Latash ML, Zatsiorsky VM (2016) Biomechanics and motor control: defining central concepts. Academic Press, New York
Latash ML, Shim JK, Smilga AV, Zatsiorsky V (2005) A central back-coupling hypothesis on the organization of motor synergies: a physical metaphor and a neural model. Biol Cybern 92:186–191
Latash ML, Scholz JP, Schöner G (2007) Toward a new theory of motor synergies. Mot Control 11:276–308
Levin MF, Dimov M (1997) Spatial zones for muscle coactivation and the control of postural stability. Brain Res 757:43–59
Levin MF, Feldman AG, Milner TE, Lamarre Y (1992) Reciprocal and coactivation commands for fast wrist movements. Exp Brain Res 89:669–677
Liddell EGT, Sherrington CS (1924) Reflexes in response to stretch (myotatic reflexes). Proc R Soc London Ser B 96:212–242
Madarshahian S, Latash ML (2021) Synergies at the level of motor units in single-finger and multi-finger tasks. Exp Brain Res 239:2905–2923
Madarshahian S, Letizi J, Latash ML (2021) Synergic control of a single muscle: the example of flexor digitorum superficialis. J Physiol 599:1261–1279
Nawab SH, Chang SS, De Luca CJ (2010) High-yield decomposition of surface EMG signals. Clin Neurophysiol 121:1602–1615
Nielsen JB (2016) Human spinal motor control. Ann Rev Neurosci 39:81–101
Nielsen JB, Kagamihara Y (1992) The regulation of disynaptic reciprocal Ia inhibition during co-contraction of antagonistic muscles in man. J Physiol 456:373–391
Nielsen JB, Pierrot-Deseilligny E (1996) Evidence of facilitation of soleus-coupled Renshaw cells during voluntary co-contraction of antagonistic ankle muscles in man. J Physiol 493:603–611
Piscitelli D, Falaki A, Solnik S, Latash ML (2017) Anticipatory postural adjustments and anticipatory synergy adjustments: preparing to a postural perturbation with predictable and unpredictable direction. Exp Brain Res 235:713–730
Reschechtko S, Latash ML (2017) Stability of hand force production: I. Hand level control variables and multi-finger synergies. J Neurophysiol 118:3152–3164
Scholz JP, Schöner G (1999) The uncontrolled manifold concept: identifying control variables for a functional task. Exp Brain Res 126:289–306
Shinohara M, Latash ML, Zatsiorsky VM (2003) Age effects on force produced by intrinsic and extrinsic hand muscles and finger interaction during MVC tasks. J Appl Physiol 95:1361–1369
Solnik S, Pazin N, Coelho C, Rosenbaum DA, Scholz JP, Zatsiorsky VM, Latash ML (2013) End-state comfort and joint configuration variance during reaching. Exp Brain Res 225:431–442
St-Onge N, Adamovich SV, Feldman AG (1997) Control processes underlying elbow flexion movements may be independent of kinematic and electromyographic patterns: experimental study and modelling. Neuroscience 79:295–316
Tilney F, Pike FH (1925) Muscular coordination experimentally studied in its relation to the cerebellum. Arch Neurol Psychiatry 13:289–334
Ting LH, Macpherson JM (2005) A limited set of muscle synergies for force control during a postural task. J Neurophysiol 93:609–613
Ting LH, McKay JL (2007) Neuromechanics of muscle synergies for posture and movement. Curr Opin Neurobiol 17:622–628
Tresch MC, Jarc A (2009) The case for and against muscle synergies. Curr Opin Neurobiol 19:601–607
Tresch MC, Cheung VC, d’Avella A (2006) Matrix factorization algorithms for the identification of muscle synergies: evaluation on simulated and experimental data sets. J Neurophysiol 95:2199–2212
Acknowledgements
We are very much grateful to Paola Contessa and Nicholas Ducey from Delsys, Inc. for the important discussions and insights.
Funding
The Galileo system used in the study was on loan from Delsys, Inc. Shirin Madarshahian was supported for two months in 2019 by a fellowship from Delsys, Inc.
Author information
Authors and Affiliations
Contributions
SM: design of the study, acquisition, analysis, and interpretation of data, writing the draft. MLL: conception and design of the study, analysis and interpretation of data, writing the draft. Both authors approved the final version of the manuscript, agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; both persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest is claimed by any of the authors.
Ethics approval
All the procedures were approved by the Office for Research Protection of the Pennsylvania State University (protocol #33393) in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Consent to participate
All of the subjects provided their informed consent based on the procedures approved by the Office for Research Protection of Pennsylvania State University.
Consent for publication
The authors and participants gave their consent to publish these data.
Additional information
Communicated by Francesco Lacquaniti.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Madarshahian, S., Latash, M.L. Reciprocal and coactivation commands at the level of individual motor units in an extrinsic finger flexor–extensor muscle pair. Exp Brain Res 240, 321–340 (2022). https://doi.org/10.1007/s00221-021-06255-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-021-06255-w