Abstract
The interaction between pain and the motor system is well-known, with past studies showing that pain can alter corticomotor excitability and have deleterious effects on motor learning. The aim of this study was to better understand the cortical mechanisms underlying the interaction between pain and the motor system. Experimental pain was induced on 19 young and healthy participants using capsaicin cream, applied on the middle volar part of the left forearm. The effect of pain on brain activity and on the corticomotor system was assessed with electroencephalography (EEG) and transcranial magnetic stimulation (TMS), respectively. Compared to baseline, resting state brain activity significantly increased after capsaicin application in the central cuneus (theta frequency), left dorsolateral prefrontal cortex (alpha frequency), and left cuneus and right insula (beta frequency). A pain-evoked increase in the right primary motor cortex (M1) activity was also observed (beta frequency), but only among participants who showed a reduction in corticospinal output (as depicted by TMS recruitment curves). These participants further showed greater beta M1-cuneus connectivity than the other participants. These findings indicate that pain-evoked increases in M1 beta power are intimately tied to changes in the corticospinal system, and provide evidence that beta M1-cuneus connectivity is related to the corticomotor alterations induced by pain. The differential pattern of response observed in our participants suggest that the effect of pain on the motor system is variable from on individual to another; an observation that could have important clinical implications for rehabilitation professionals working with pain patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pain is a rapidly growing area of research, and the last years have shown huge advancement in our understanding of its neurophysiological process. The development of neuroimagery techniques have led to the discovery that pain perception is intimately linked to the activation of a complex cerebral network comprised, among other things, of the primary somatosensory cortex (S1) and the secondary somatosensory cortex (S2), the anterior cingulate cortex (ACC) and the insula (IC) (Apkarian et al. 2005; Forster and Handwerker 2014; Nakata et al. 2014).
A few neuroimagery studies have also reported an increase in the activity of the primary motor cortex (M1) in the presence of experimental pain (Apkarian et al. 2000; Tracey et al. 2000; Burns et al. 2016). Stancák et al. demonstrated, using electroencephalography (EEG), that the application of a short-lasting painful heat stimuli on the hand decreased the β activity of the sensorimotor cortex (Stancák et al. 2007). Given the inhibitory role that β waves have on the motor cortex (Pogosyan et al. 2009), the decrease in M1 β activity noted by Stancák and colleagues suggests that the presence of a brief nociceptive stimulus could prime the motor brain regions (reduced inhibition), possibly to facilitate motor withdrawal responses. As pointed out, the results obtained by Stancák and colleagues were obtained following the application of brief/escapable, nociceptive stimuli and it remains uncertain whether the same pattern of results would be obtained with longer/unavoidable nociceptive stimulations.
The observations made with neuroimagery techniques are consistent with the results of studies performed with transcranial magnetic stimulation (TMS). TMS studies have shown that experimental pain stimulation can alter the excitability of the corticomotor system (Farina et al. 2001; Valeriani et al. 2001). However, contrary to the study by Stancák et al. (that suggest a priming of the motor cortex in the presence of pain), TMS studies generally report reduced corticospinal excitability following nociceptive stimuli (Boudreau et al. 2007; Mercier and Leonard 2011; Schabrun and Hodges 2012; Schabrun et al. 2013; Rittig-Rasmussen et al. 2014). Some researchers have suggested that these corticomotor effects could explain the negative impact that pain can have on motor learning (Boudreau et al. 2007; Rittig-Rasmussen et al. 2014). Supporting this are the results of Rittig-Rasmussen et al. (Rittig-Rasmussen et al. 2014) who have observed that the change in corticospinal excitability (increased motor-evoked potential [MEP] amplitudes) noted following upper trapezius training was completely blocked by a hypertonic muscle saline injection, with the effect being apparent up to 7 days post-training.
Interestingly, several neuroimagery and neurostimulation studies have shown that patients suffering from clinical pain conditions show changes in cortical representation at the M1 level. For example, in patients suffering from complex regional pain syndrome (CRPS) and from phantom limb pain, researchers have reported reduced cortical representation of the affected limb (Karl et al. 2001; Krause et al. 2006). Although compelling, these studies remain correlational and it is impossible to know if the neuroplastic changes in M1 are directly caused by pain. The use of an experimental pain paradigm, in which the researchers can manipulate the presence of pain, would make it possible to address this question and determine whether pain is causally linked to corticomotor changes.
In this study, TMS and EEG were used concomitantly to better understand the effect of pain on the motor system. More specifically, the objectives were to evaluate the effect of a prolonged/inescapable nociceptive stimulation on TMS recruitment curves [a measure believed to reflect the strength of the corticospinal projections (Devanne et al. 1997; Abbruzzese and Trompetto 2002)] and on the pattern of EEG activity of the motor brain regions. A second objective was to determine if these potential changes in the TMS recruitment curve and EEG activity could be related to changes in functional connectivity between M1 and other brain regions implicated in the perception of pain.
Materials and methods
Participants
Nineteen healthy, right handed adults (12 women and 7 men; mean age 29 ± 7 years old) participated in the study. To be included in the study, participants had to be aged over 18 years and be pain-free (absence of painful health condition and no pain upon testing). For security reasons, individuals with neurological disorders, metal implants in the skull, a pacemaker or neurostimulator, epilepsy or pregnant were excluded from the study. Participants were asked to refrain from consuming caffeine for 6 h before testing, and tobacco products for 2 h before testing. The research protocol was approved by the ethics committee of the Research Centre on Aging (Sherbrooke, Quebec, Canada) and each participant provided informed written consent before participating in the study.
Transcranial magnetic stimulation (TMS)
Magnetic stimuli were delivered by a 70 mm figure-eight coil connected to a Magstim 200 (Magstim Co., Dyfed, UK). Participants sat in a comfortable chair and two Ag/AgCl surface recording electrodes (1 cm2 recording area) were positioned over their left first dorsal interosseous (FDI) muscle to record motor-evoked potentials (MEP). Electromyographic signals, elicited by the magnetic stimuli, were amplified and filtered (bandwidth 200 Hz to 2 kHz) with a CED 1902 amplifier (Cambridge Electronic Design Limited, Cambridge, UK), and digitized at a sampling rate of 10 kHz using a Power 1401 mk II interface and Spike 2 software (version 7.10; Cambridge Electronic Design Limited, Cambridge, UK).
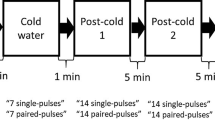
With the coil held ~45° in the mid-sagittal plane, the approximate location of the FDI muscle on the right hemisphere was explored in 1-cm step until reliable MEP could be evoked in the FDI. The optimal location for eliciting MEP in the FDI was found (hotspot). This site was then marked on the scalp of the participants with a marker to ensure consistent coil positioning. Throughout the experiment, the experimenter frequently reassessed the coil position to ensure that it remained over the optimal stimulation site. At this point, stimulations of varying intensities were sent to determine the resting motor threshold (rMT), defined for each participant as the minimal intensity of stimulation capable of eliciting MEPs of at least 50 µV in 50% of the trials with the FDI at rest (no muscle contraction). Then, 4 blocks of 10 stimulations were provided randomly to participants (delay between each stimulation = 5 to 8 s), with the stimulation in each block given at the same intensity (i.e., 90, 110, 130, and 150% of rMT). The peak-to-peak amplitude of MEP responses were measured off-line and averaged for each participant to derive mean values. The slope of the recruitment curve (describing the relationship between MEP amplitude and TMS intensity) was then calculated using hierarchical linear modeling (HLM) (Roberts et al. 2010).
Electroencephalography (EEG)
EEG activity was recorded at rest using a 32-channel EEG acquisition system (Brain Products GmBh, Munich, Germany) with electrodes positioned according to the international 10–20 system. Data were recorded at 500 Hz for 5 min using FCz reference and keeping all electrode impedances below 5 kΩ. Eye blinks and motion artifacts were removed from the data using independent component analysis (ICA) denoising (Brain Vision Analyzer, Brain Products GmbH, Munich, Germany). Data were then re-referenced to the common average.
For each participant, 15 non-overlapping, 2-s segments without artifacts were randomly selected and decomposed in eight frequency bands: δ (delta: 1.5–4 Hz), θ (theta: 4–8 Hz), α1 (alpha 1: 8–10 Hz), α2 (alpha 2: 10–13 Hz), β1 (beta 1: 13–21 Hz), β2 (beta 2: 21–30 Hz), β3 (beta 3: 30–60 Hz) and ω (omega >60 Hz). For each segment, intracranial source current densities were then computed using sLORETA software (Pascual-Marqui 2002), yielding sources in 6239 5 × 5 × 5 mm3 cortical grey matter voxels in standard MNI space (Fonov et al. 2011). sLORETA allows the localization of spatially distributed sources of activity without a priori on their number, which is well suited in the context of pain (Apkarian et al. 2005; Tracey and Mantyh 2007; Schweinhardt and Bushnell 2010). Current density maps were then averaged across segments for each subject and condition (i.e., baseline and pain condition).
Capsaicin application
After the evaluation of baseline TMS and EEG measures, experimental pain was induced by a 1% capsaicin cream. More specifically, 0.06 ml of capsaicin was applied on the middle volar part of the left forearm in a perimeter of 4 × 4 cm. Capsaicin-induced pain was evaluated by the participants using a visual analogue scale (VAS; 0 = “no pain”, 10 = “the worst imaginable pain”), every 5 min until the pain sensation stabilized (i.e., when participants rated same intensity of pain in 2 consecutive VAS pain measures). Once the pain became stable, EEG and TMS measures were assessed again (see Fig. 1).
Statistical analysis
Paired-sample t tests were used to determine if there was a difference between the baseline and pain condition for the HLM values. Changes in current density power (EEG activity) between the baseline and pain condition were assessed using paired-sample t-tests across subjects, independently for each frequency band and each voxel. Statistical significance was assessed through statistical nonparametric mapping using 5000 randomizations to account for multiple comparisons. A threshold on the t-statistic corresponding to p < 0.05 was used to uncover pain-evoked activation maps and identify regions of the brain displaying changes in activity between the rest and pain conditions.
Because the analyses revealed no consistent changes in TMS measures and EEG activity between the baseline and pain condition (see Results section below), separate functional connectivity analyses were conducted in participants who showed a reduction in corticospinal output and an increase in M1 β activity (group 1), and in participants who did not (group 2). For each group, linear lagged connectivity was assessed in the β band frequency using sLORETA software between M1 (region of interest) and other brain regions in which an increase in activity was observed during the pain condition. These functional connectivity analyses allowed us to evaluate if the activation of M1 was related to an interaction with other brain structures also activated in the presence of pain (Apkarian et al. 2000; Tracey et al. 2000).
Results
Pain assessment
Every participant experienced pain following capsaicin application (mean pain intensity = 4 ± 2). On average, 42 min were required after capsaicin application before the pain stabilized.
Effect of experimental pain on TMS recruitment curves
TMS recruitment curves obtained before and after capsaicin application are presented in Fig. 2. As can be seen from this figure, pain did not affect corticospinal output, as evidenced by the comparable TMS recruitment curves obtained for the baseline and pain condition. The absence of difference between the two conditions was confirmed by the statistical analysis, with the paired-sample t test showing no difference in HLM slope values between the baseline and pain condition (p = 0.26). Pearson correlational analyses showed that there were no relationships between the change in the slope of the recruitment curve and the time needed for pain to reach a plateau (r = −0.02; p = 0.92) and between the change in the slope of the recruitment curve and the intensity of pain reported by the participants (r = −0.21; p = 0.36).
TMS recruitment curves obtained before and during pain. HLM analyses were used to estimate the slope of the recruitment curves of each participant. There was no change in the HLM slope value between the baseline and the pain condition in the total sample (n = 19), indicating that pain had no consistent effect on corticospinal output
Effect of experimental pain on brain activity
Source localization analyses conducted to compare brain responses between the baseline and pain condition revealed a significant increase in brain activity across the central cuneus (x = 0, y = −85, z = 10 at theta frequency), the left dorsolateral prefrontal cortex (DFPLC) (x= −45, y = 30, z = 35 at alpha frequency), and the left cuneus (x = −20, y = −90, z = 35) and right insula (x = 35, y = −5, z = 20 both at the beta frequency) while participants were in the pain condition (all ts >4.40, corresponding to p < 0.05). No changes were noted in other brain regions, including M1 (all p values >0.05).
Between-group analyses
Careful examination of the data revealed that about two-thirds of the participants (n = 12) showed a decrease in corticospinal output (reduced TMS recruitment curve slope) during the pain condition while the other third (n = 7) showed an increase in corticospinal output (increased TMS recruitment curve slope; see Figs. 3a, b, 4). These observations brought us to evaluate and to compare the changes in EEG brain activity and functional connectivity between these two groups of participants.
Delta scores, reflecting the change in the slope of the recruitment curves (RC). Delta scores were calculated for each participant by subtracting the HLM slope value obtained during the pain condition from the HLM slope value obtained during baseline condition. Negative delta scores indicate reduced recruitment curve slopes, while positive scores indicate an increase in the slope of the recruitment curve
The between-group analysis first revealed that, compared to participants who showed an increase in corticospinal output, participants who showed a decrease in corticospinal output also showed greater right M1 beta frequency activity (x = 35, y = −15, z = 50; t = 4.69, p = 0.049) in the pain condition (see Fig. 5). Importantly, this group difference was absent at baseline (all ts <4.80, p > 0.48). Between-group comparisons, looking at changes in EEG functional connectivity, showed that, compared to participants who showed an increase in corticospinal output, those who showed a decrease demonstrated greater pain-related beta M1-cuneus connectivity (t = 3.58, p = 0.03). Again, these between group differences in beta M1-cuneus connectivity were not found at baseline (t = 3.73, p = 0.73). No other connectivity change was observed (all p values >0.05).
Discussion
The current study’s objective was to better understand the corticomotor changes induced by pain. More specifically, we wanted to determine if a prolonged/inescapable nociceptive stimulation pain, induced with a capsaicin cream, could modify TMS recruitment curves as well as EEG activity of the motor cortex, and if these eventual alterations could be associated to functional connectivity changes. Our analyses revealed that capsaicin pain produced variable effects, with approximately two-thirds of participants showing a reduced TMS recruitment curve slope. Participants who showed this type of decrease also showed an increase in M1 β activity.
Effect of pain on cortical representation and corticospinal output
In the past years, many studies have revealed the presence of functional reorganizations in the somatosensory and motor system of pain patients. For example, Krause et al. observed that patients with complex regional pain syndrome (CRPS) had a smaller corticomotor representation of the affected limb, compared to pain-free participants (Krause et al. 2006). Flor et al. reported similar changes in the primary somatosensory cortex (S1) in people suffering from phantom pain (Flor 2003). Interestingly, researchers observed the presence of a positive correlation between pain intensity and the amplitude of cortical reorganization in amputee patients, suggesting that these neuroplastic changes could play an important role in the physiopathology of persistent pain (Flor et al. 1995).
The idea that cortical reorganization could play an important role in the physiopathology of chronic pain was reinforced by Maihofner et al. and Pleger et al. who observed a normalization of the cortical changes in CRPS patients after successful treatment, once pain subsided (Maihofner et al. 2004; Pleger et al. 2005). The results of Maihofner et al. and Pleger et al. support the idea that pain could drive cortical reorganization; however, the ultimate way to confirm the presence of a causal relationship between pain and cortical changes is to experimentally manipulate the presence of pain, as it is the case in this study. Our results show that pain can, indeed, drive changes in the corticomotor system, but that its effect is not uniform across all individuals.
Nevertheless, we must remember that the results obtained from experimental pain paradigm cannot be directly generalized to clinical pain populations. It should also be noted that the effect of pain on the motor system can vary depending on the duration of the painful stimulus (phasic vs tonic pain), the submodality (deep vs superficial pain), and the location (proximal vs distal pain) (Valeriani et al. 1999, 2001; Farina et al. 2001; Le Pera et al. 2001; Cheong et al. 2003; Svensson et al. 2003; Mercier and Leonard 2011). Replicating the present results with different experimental pain paradigms and pursuing research in pain populations is essential before any final conclusions can be made.
Effect of pain on EEG activity of the motor cortex
Several neuroimaging studies have shown that experimental pain can affect the activity of the motor cortex (Apkarian et al. 2000; Tracey et al. 2000; Burns et al. 2016). For the most part, these studies were done using functional magnetic resonance imaging (fMRI). Although useful—in particular because of its ability to measure changes in deep areas of the brain—it is important to remember that fMRI BOLD responses reflect changes in cerebral blood flow, cerebral blood volume and cerebral metabolic rate of oxygen following neural activation (Fox and Raichle 1986; Uludag et al. 2009; Attwell et al. 2010). As such, changes in BOLD can, at best, be related to changes in neural activity and cannot be interpreted specifically in terms of excitatory (increase in the activity of excitatory neurons) or inhibitory (increase in the activity of inhibitory neurons) activity. Contrary to fMRI, EEG directly measures the neuroelectric activity of brain cells, allowing a better characterization of neuronal changes (Aine 1995). In this study, the EEG analyses have revealed that the majority of participants showed increased contralateral M1 β frequency activity during pain, suggesting that nociception increases the inhibitory activity in this area (Pogosyan et al. 2009). The biological reasons for these cortical changes remain hypothetical. A possible explanation is that increased β activity could force the injured individual to limit his movements, to promote healing. However, in certain cases, this inhibitory effect could be detrimental, for example by interfering with motor learning and rehabilitation (Boudreau et al. 2007; Bouffard et al. 2014).
In the past years, accumulating evidence stemming from paired-pulse TMS studies has suggested that chronic pain populations display changes in GABA-mediated intracortical inhibition (see for instance Parker et al. (2016) for a review). Perhaps the most compelling observations are the ones made by Lefaucheur and colleagues (Lefaucheur et al. 2006). In this study, Lefaucheur and colleagues observed that (1) neuropathic pain patients had reduced intracortical inhibition, when compared to age-matched healthy controls, (2) application of high-frequency (10 Hz) repetitive TMS (rTMS) in these pain patients increased intracortical inhibition, and (3) there was a significant association between the extent of pain relief and the increase in intracortical inhibition observed following the application of rTMS. Changes in GABA-mediated short intracortical inhibition (SICI) have also been documented with experimental pain paradigms (Fierro et al. 2010; Schabrun and Hodges 2012). Results from these studies indicate that the effect of experimental pain on SICI may depend on the nature/location of the nociceptive stimulus; while Fierro et al. (2010) observed reduced SICI following a topical capsaicin application (superficial cutaneous pain), Schabrun and Hodges (2012) reported increased SICI following injection of a hypertonic saline solution (deep muscle pain). Changes in intracortical facilitation (ICF) were also noted by Schabrun and Hodges (2012), but not by Fiero et al. (2010). These findings help to better understand the role played by intracortical circuits and remind researchers that the effect of pain on the corticomotor system likely varies depending on the type of pain.
The increase in β power observed in the majority of our participants contrast with the results of Stancák and colleagues, who showed that thermode induced pain decreased M1 β activity (Stancák et al. 2007). This discrepancy could be explained by the fact that prolonged pain (e.g. capsaicin) and brief pain (e.g. thermode) stimulation may foster the emergence of different motor strategies. Whereas immobilization can be a successful strategy in the former case, this same response could be detrimental in the second case, when it is possible for the individual to remove the body part away from the painful stimuli. Decreasing β activity during brief/escapable nociceptive stimulation could promote movement and help the individual avoid potential threats.
Associations between M1 β power and GABA concentration have been observed by Baumgarten and colleagues (2016). Similarly, Farzan et al. (2013) noted that the duration of the silent period [a TMS measure mediated by GABA receptors (Abbruzzese and Trompetto 2002; Jono et al. 2016)] is related to β oscillations. Taken together, these observations suggest that the changes observed in corticospinal output in some of our participants could be linked to changes in GABA activity.
Effect of pain on other brain areas
The EEG analysis revealed an increase of the activity of the insula, DFPLC and cuneus in the pain condition in all participants, when compared to baseline. The role of the insula and DFPLC in pain perception and modulation has been well documented in previous pain studies (Rainville et al. 2000; Borckardt et al. 2007); however, the activation of the cuneus in the pain condition is more unexpected. A previous study, from our research group, did suggest that a brain area adjacent to the cuneus could play a significant role in the perception of pain (Goffaux et al. 2014). In this past study, we observed that individuals who showed increased activity in the precuneus in the presence of experimental pain also showed the promptest response to pain. Traditionally linked to the treatment of visual information (Corbetta et al. 1995; Nobre et al. 2003), the cuneus also plays an important role in the integration of sensory information, as well as cognitive processes such as attention, learning and memory (Cabeza et al. 2002; Makino et al. 2004).
The functional connectivity analyzes, done on the subgroup of participants for whom pain reduced corticospinal output, further highlight the potential role that the cuneus could play in pain processes. These analyses have shown that the application of a capsaicin cream increases the functional connectivity between the motor cortex and the cuneus in individuals who show a reduced TMS recruitment curve slope. These results reinforce the role that the cuneus could play as a significant brain area for the integration of sensory and attentional information. This integrative function of the cuneus makes it an ideal cerebral structure, capable of modulating the activity and organization of the motor cortex based on the ascending sensory information and on the context in which the individual is placed and asked to interact.
Limits
The most important limit of this study probably relates to the inconsistent effect produced by pain on the corticomotor system. Indeed, it should be reminded that the most compelling findings (i.e., increased M1 β activity and reduced corticospinal output) were found in a subsample of participants. Future studies need to be conducted to determine if these results can be consistently reproduced and validate that the observed TMS and EEG changes are not spurious effects only. An additional limitation concerns the absence of control group. Although the TMS and EEG measures have been proven to be reliable (Cacchio et al. 2009; Cannon et al. 2012; Ngomo et al. 2012), the addition of a control group would have been an important asset for the study to document the stability of the TMS and EEG measures over time. Finally, it should be noted that the effect of pain on TMS and EEG measures was investigated only once (i.e., when pain stabilized). Again, futures studies, looking into the long-term effects are warranted.
Conclusion
In conclusion, our results show that tonic experimental pain increases M1 β activity in certain individuals, and that this increase in β activity is intimately tied to corticomotor and functional connectivity changes. These observations remind us that the cerebrum works as an integrated system of circuits and that certain brain areas, other than those classically involved in pain perception and modulation can be affected by nociceptive stimulations. The differential pattern of response observed in our participants suggest that the effect of pain on the motor system is variable from an individual to another; an observation that could have important clinical implications for rehabilitation professionals working with pain patients.
References
Abbruzzese G, Trompetto C (2002) Clinical and research methods for evaluating cortical excitability. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc 19:307–321
Aine CJ (1995) A conceptual overview and critique of functional neuroimaging techniques in humans: I. MRI/FMRI and PET. Crit Rev Neurobiol 9:229–309
Apkarian AV, Gelnar PA, Krauss BR, Szeverenyi NM (2000) Cortical responses to thermal pain depend on stimulus size: a functional MRI study. J Neurophysiol 83:3113–3122
Apkarian AV, Bushnell MC, Treede RD, Zubieta JK (2005) Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9:463–484
Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA (2010) Glial and neuronal control of brain blood flow. Nature 468:232–243
Baumgarten TJ, Oeltzschner G, Hoogenboom N, Wittsack HJ, Schnitzler A, Lange J (2016) Beta peak frequencies at rest correlate with endogenous GABA+/Cr concentrations in sensorimotor cortex areas. PLoS One 11:e0156829
Borckardt JJ, Smith AR, Reeves ST, Weinstein M, Kozel FA, Nahas Z, Shelley N, Branham RK, Thomas KJ, George MS (2007) Fifteen minutes of left prefrontal repetitive transcranial magnetic stimulation acutely increases thermal pain thresholds in healthy adults. Pain Res Manag 12:287–290
Boudreau S, Romaniello A, Wang K, Svensson P, Sessle BJ, Arendt-Nielsen L (2007) The effects of intra-oral pain on motor cortex neuroplasticity associated with short-term novel tongue-protrusion training in humans. Pain 132:169–178
Bouffard J, Bouyer LJ, Roy JS, Mercier C (2014) Tonic pain experienced during locomotor training impairs retention despite normal performance during acquisition. J Neurosci 34:9190–9195
Burns E, Chipchase LS, Schabrun SM (2016) Primary sensory and motor cortex function in response to acute muscle pain: a systematic review and meta-analysis. Eur J Pain 20:1203–1213
Cabeza R, Dolcos F, Graham R, Nyberg L (2002) Similarities and differences in the neural correlates of episodic memory retrieval and working memory. Neuroimage 16:317–330
Cacchio A, Cimini N, Alosi P, Santilli V, Marrelli A (2009) Reliability of transcranial magnetic stimulation-related measurements of tibialis anterior muscle in healthy subjects. Clin Neurophysiol 120:414–419
Cannon RL, Baldwin DR, Shaw TL, Diloreto DJ, Phillips SM, Scruggs AM, Riehl TC (2012) Reliability of quantitative EEG (qEEG) measures and LORETA current source density at 30 days. Neurosci Lett 518:27–31
Cheong JY, Yoon TS, Lee SJ (2003) Evaluations of inhibitory effect on the motor cortex by cutaneous pain via application of capsaicin. ElectromyogrClin Neurophysiol 43:203–210
Corbetta M, Shulman GL, Miezin FM, Petersen SE (1995) Superior parietal cortex activation during spatial attention shifts and visual feature conjunction. Science 270:802–805
Devanne H, Lavoie BA, Capaday C (1997) Input–output properties and gain changes in the human corticospinal pathway. Exp Brain Res 114:329–338
Farina S, Valeriani M, Rosso T, Aglioti S, Tamburin S, Fiaschi A, Tinazzi M (2001) Transient inhibition of the human motor cortex by capsaicin-induced pain. A study with transcranial magnetic stimulation. NeurosciLett 314:97–101
Farzan F, Barr MS, Hoppenbrouwers SS, Fitzgerald PB, Chen R, Pascual-Leone A, Daskalakis ZJ (2013) The EEG correlates of the TMS-induced EMG silent period in humans. Neuroimage 83:120–134
Fierro B, De Tommaso M, Giglia F, Giglia G, Palermo A, Brighina F (2010) Repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex (DLPFC) during capsaicin-induced pain: modulatory effects on motor cortex excitability. Exp Brain Res 203:31–38
Flor H (2003) Cortical reorganisation and chronic pain: implications for rehabilitation. J Rehabil Med 66–72
Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, Larbig W, Taub E (1995) Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature 375:482–484
Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL (2011) Unbiased average age-appropriate atlases for pediatric studies. Neuroimage 54:313–327
Forster C, Handwerker HO (2014) Frontiers in neuroscience central nervous processing of itch and pain. In: Carstens E, Akiyama T (eds) Itch: mechanisms and treatment, CRC Press/Taylor & Francis FF(c) 2014 by Taylor & Francis Group, LLC, Boca Raton
Fox PT, Raichle ME (1986) Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci USA 83:1140–1144
Goffaux P, Girard-Tremblay L, Marchand S, Daigle K, Whittingstall K (2014) Individual differences in pain sensitivity vary as a function of precuneus reactivity. Brain Topogr 27:366–374
Jono Y, Iwata Y, Mizusawa H, Hiraoka K (2016) Change in excitability of corticospinal pathway and GABA-mediated inhibitory circuits of primary motor cortex induced by contraction of adjacent hand muscle. Brain Topogr
Karl A, Birbaumer N, Lutzenberger W, Cohen LG, Flor H (2001) Reorganization of motor and somatosensory cortex in upper extremity amputees with phantom limb pain. J Neurosci 21:3609–3618
Krause P, Forderreuther S, Straube A (2006) TMS motor cortical brain mapping in patients with complex regional pain syndrome type I. Clin Neurophysiol 117:169–176
Le Pera D, Graven-Nielsen T, Valeriani M, Oliviero A, Di Lazzaro V, Tonali PA, Arendt-Nielsen L (2001) Inhibition of motor system excitability at cortical and spinal level by tonic muscle pain. Clin Neurophysiol 112:1633–1641
Lefaucheur JP, Drouot X, Menard-Lefaucheur I, Keravel Y, Nguyen JP (2006) Motor cortex rTMS restores defective intracortical inhibition in chronic neuropathic pain. Neurology 67:1568–1574
Maihofner C, Handwerker HO, Neundorfer B, Birklein F (2004) Cortical reorganization during recovery from complex regional pain syndrome. Neurology 63:693–701
Makino Y, Yokosawa K, Takeda Y, Kumada T (2004) Visual search and memory search engage extensive overlapping cerebral cortices: an fMRI study. Neuroimage 23:525–533
Mercier C, Leonard G (2011) Interactions between pain and the motor cortex: Insights from research on phantom limb pain and complex regional pain syndrome. Physiother Can 63:305–314
Nakata H, Sakamoto K, Kakigi R (2014) Meditation reduces pain-related neural activity in the anterior cingulate cortex, insula, secondary somatosensory cortex, and thalamus. Front Psychol 5:1489
Ngomo S, Leonard G, Moffet H, Mercier C (2012) Comparison of transcranial magnetic stimulation measures obtained at rest and under active conditions and their reliability. J Neurosci Methods 205:65–71
Nobre AC, Coull JT, Walsh V, Frith CD (2003) Brain activations during visual search: contributions of search efficiency versus feature binding. Neuroimage 18:91–103
Parker RS, Lewis GN, Rice DA, McNair PJ (2016) Is motor cortical excitability altered in people with chronic pain? A systematic review and meta-analysis. Brain Stimul 9:488–500
Pascual-Marqui RD (2002) Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol, 24(Suppl D):5–12 Spain
Pleger B, Tegenthoff M, Ragert P, Forster AF, Dinse HR, Schwenkreis P, Nicolas V, Maier C (2005) Sensorimotor retuning in complex regional pain syndrome parallels pain reduction. AnnNeurol 57:425–429
Pogosyan A, Gaynor LD, Eusebio A, Brown P (2009) Boosting cortical activity at beta-band frequencies slows movement in humans. Curr Biol CB 19:1637–1641
Rainville P, Duncan G, Bushnell M (2000) Représentation cérébrale de l’expérience subjective de la douleur chez l’homme. Médecine/sciences 16:519–527
Rittig-Rasmussen B, Kasch H, Fuglsang-Frederiksen A, Svensson P, Jensen TS (2014) The role of neuroplasticity in experimental neck pain: a study of potential mechanisms impeding clinical outcomes of training. Man Ther 19:288–293
Roberts DR, Ramsey D, Johnson K, Kola J, Ricci R, Hicks C, Borckardt JJ, Bloomberg JJ, Epstein C, George MS (2010) Cerebral cortex plasticity after 90 days of bed rest: data from TMS and fMRI. Aviat Space Environ Med 81:30–40
Schabrun SM, Hodges PW (2012) Muscle pain differentially modulates short interval intracortical inhibition and intracortical facilitation in primary motor cortex. J Pain 13:187–194
Schabrun SM, Jones E, Kloster J, Hodges PW (2013) Temporal association between changes in primary sensory cortex and corticomotor output during muscle pain. Neuroscience 235:159–164
Schweinhardt P, Bushnell MC (2010) Pain imaging in health and disease—how far have we come? J Clin Invest 120:3788–3797
Stancák A, Polácek H, Vrána J, Mlyná J (2007) Cortical oscillatory changes during warming and heating in humans. Neuroscience 147:842–852
Svensson P, Miles TS, McKay D, Ridding MC (2003) Suppression of motor evoked potentials in a hand muscle following prolonged painful stimulation. EurJ Pain 7:55–62
Tracey I, Mantyh PW (2007) The cerebral signature for pain perception and its modulation. Neuron 55:377–391
Tracey I, Becerra L, Chang I, Breiter H, Jenkins L, Borsook D, Gonzalez RG (2000) Noxious hot and cold stimulation produce common patterns of brain activation in humans: a functional magnetic resonance imaging study. Neurosci Lett 288:159–162
Uludag K, Muller-Bierl B, Ugurbil K (2009) An integrative model for neuronal activity-induced signal changes for gradient and spin echo functional imaging. Neuroimage 48:150–165
Valeriani M, Restuccia D, Di Lazzaro V, Oliviero A, Profice P, Le Pera D, Saturno E, Tonali P (1999) Inhibition of the human primary motor area by painful heat stimulation of the skin. Clin Neurophysiol 110:1475–1480
Valeriani M, Restuccia D, Di Lazzaro V, Oliviero A, Le Pera D, Profice P, Saturno E, Tonali P (2001) Inhibition of biceps brachii muscle motor area by painful heat stimulation of the skin. ExpBrain Res 139:168–172
Acknowledgements
The authors gratefully acknowledge Antoine Guillerand and Mathieu Hamel for their help with data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Research funding
This work was supported by a grant from Natural Sciences and Engineering Research Council of Canada (NSERC).
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Martel, M., Harvey, MP., Houde, F. et al. Unravelling the effect of experimental pain on the corticomotor system using transcranial magnetic stimulation and electroencephalography. Exp Brain Res 235, 1223–1231 (2017). https://doi.org/10.1007/s00221-017-4880-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-017-4880-0