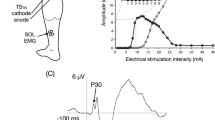
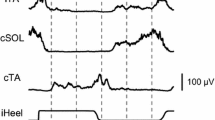
Abstract
Recent studies have shown that afferents arising from muscle receptors located on one side can affect the activity of muscles on the contralateral side. In animal preparations, evidence supports that afferent pathways originating from one limb converge onto interneurons mediating disynaptic reciprocal Ia inhibition of the opposite limb. This study was designed to investigate whether this pathway is similar in humans to that described in animals. Thirteen healthy volunteers participated in one of two experiments. In experiment 1, the effects of ipsilateral posterior tibial nerve (iPTN) stimulation were assessed on the reciprocal Ia inhibition of the contralateral soleus (cSOL) motoneuronal pool (n = 8). Across all participants, iPTN stimulation intensity was 1.69 ± 0.3 × Motor Threshold (MT) and contralateral common peroneal (cCPN) stimulation intensity was 0.86 ± 0.16 × MT. iPTN and cCPN stimulation were delivered separately or in combination and changes in the ongoing electromyography (EMG) quantified. In experiment 2, the amplitude of a test SOL H-reflex elicited by contralateral PTN (cPTN) stimulation was quantified following iPTN, cCPN or iPTN + cCPN nerve stimulation (n = 5). Intensities used during the H-reflex conditioning experiment were 1.79 ± 0.4 × MT for the iPTN stimulation and 0.88 ± 0.16 × MT for cCPN stimulation. Across all participants, the onset of the cSOL EMG suppression was 42 ± 4, 44 ± 3 and 44 ± 3 ms for iPTN, cCPN and iPTN + cCPN conditions, respectively. The inhibition from the combined iPTN and cCPN stimulation was significantly greater compared to the algebraic sum of their separate effects. When conditioning the cSOL H-reflex, the ISI between the test cPTN and the iPTN or cCPN stimulus was 5.4 ± 0.5 and 2.6 ± 0.5, respectively. The combined stimulation induced a significantly greater inhibition compared to their separate effects. These data provide evidence of convergence on common inhibitory interneurons by muscle afferents activated by iPTN and cCPN stimulation during sitting. Since the inhibition elicited by cCPN stimulation is known to be mediated by the disynaptic Ia inhibitory pathway, this suggests that the crossed inhibition of cSOL motoneurones elicited by muscle afferents from the ipsilateral plantarflexor muscles is at least partly mediated by Ia inhibitory interneurons in the contralateral human spinal cord. This is similar to what has been observed in the cat.



Similar content being viewed by others
References
Bannatyne BA, Liu TT, Hammar I, Stecina K, Jankowska E, Maxwell DJ (2009) Excitatory and inhibitory intermediate zone interneurons in pathways from feline group I and II afferents: differences in axonal projections and input. J Physiol 587:379–399. doi:10.1113/jphysiol.2008.159129
Biro Z, Hill RH, Grillner S (2008) The activity of spinal commissural interneurons during fictive locomotion in the lamprey. J Neurophysiol 100:716–722. doi:10.1152/jn.90206.2008
Cram J, Kasman G, Holtz J (1998) Introduction to surface electromyography. Aspen, Gaithersburg
Crone C, Hultborn H, Jespersen B (1985) Reciprocal Ia inhibition from the peroneal nerve to soleus motoneurones with special reference to the size of the test reflex. Exp Brain Res 59:418–422
Crone C, Hultborn H, Jespersen B, Nielsen J (1987). Reciprocal Ia inhibition between ankle flexors and extensors in man. J Physiol (Lond) 389, 163–185.
Dietz V, Horstmann GA, Berger W (1989) Interlimb coordination of leg-muscle activation during perturbation of stance in humans. J Neurophysiol 62:680–693
Eccles RM, Lundberg A (1957) Spatial facilitation in the direct inhibitory pathway. Nature 179:1305–1306
Hanna-Boutros B, Sangari S, Karasu A, Giboin LS, Marchand-Pauvert V (2014) Task-related modulation of crossed spinal inhibition between human lower limbs. J Neurophysiol 111:1865–1876. doi:10.1152/jn.00838.2013
Harrison PJ, Zytnicki D (1984) Crossed actions of group I muscle afferents in the cat. J Physiol (Lond) 356: 263–273. doi:10.1113/jphysiol.1984.sp015463
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70
Hultborn H, Udo M (1972) Convergence in the reciprocal Ia inhibitory pathway of excitation from descending pathways and inhibition from motor axon collaterals. Acta Physiol Scand 84:95–108
Jankowska E (2001) Spinal interneuronal systems: identification, multifunctional character and reconfigurations in mammals. [Review]. J Physiol (Lond) 533:31–40
Jankowska E, Lundberg A (1981) Interneurones in the spinal cord. TINS 4(9):230–233
Jankowska E, McCrea DA (1983) Shared reflex pathways from Ib tendon organ afferents and Ia muscle spindle afferents in the cat. J Physiol 338:99–111
Jankowska E, Edgley SA, Krutki P, Hammar I (2005a) Functional differentiation and organization of feline midlumbar commissural interneurones. J Physiol (Lond) 565:645–658
Jankowska E, Krutki P & Matsuyama K (2005b) Relative contribution of Ia inhibitory interneurones to inhibition of feline contralateral motoneurones evoked via commissural interneurones. J Physiol (Lond) 568:617–628
Jankowska E, Bannatyne BA, Stecina K, Hammar I, Cabaj A, Maxwell DJ (2009) Commissural interneurons with input from group I and II muscle afferents in feline lumbar segments: neurotransmitters, projections and target cells. J Physiol 587:401–418. doi:10.1113/jphysiol.2008.159236
Kiehn O (2006) Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci 29:279–306. doi:10.1146/annurev.neuro.29.051605.112910
Kjaerulff O, Kiehn O (1997) Crossed rhythmic synaptic input to motoneurons during selective activation of the contralateral spinal locomotor network. J Neurosci 17:9433–9447
Meunier S, Pierrot-Deseilligny E, Simonetta M (1993) Pattern of monosynaptic heteronymous Ia connections in the human lower limb. Exp Brain Res 96:534–544
Petersen N, Morita H, Nielsen J (1998) Evaluation of reciprocal inhibition of the soleus H-reflex during tonic plantar flexion in man. J Neurosci Methods 84:1–8
Pierrot-Deseilligny E, Burke D (2005) The circuitry of the human spinal cord: Its role in motor control and movement disorders. Cambridge University Press, Cambridge
Pierrot-Deseilligny E, Morin C, Bergego C, Tankov N (1981) Pattern of group I fibre projections from ankle flexor and extensor muscles in man. Exp Brain Res 42:337–350
Stevenson AJ, Geertsen SS, Andersen JB, Sinkjaer T, Nielsen JB, Mrachacz-Kersting N (2013) Interlimb communication to the knee flexors during walking in humans. J Physiol (Lond) 591:4921–4935
Stubbs PW, Mrachacz-Kersting N (2009) Short-latency crossed inhibitory responses in the human soleus muscle. J Neurophysiol 102:3596–3605. doi:10.1152/jn.00667.2009
Stubbs PW, Nielsen JF, Sinkjaer T, Mrachacz-Kersting N (2011a) Crossed spinal soleus muscle communication demonstrated by H-reflex conditioning. Muscle Nerve 43:845–850. doi:10.1002/mus.21964
Stubbs PW, Nielsen JF, Sinkjaer T, Mrachacz-Kersting N (2011b) Phase modulation of the short-latency crossed spinal response in the human soleus muscle. J Neurophysiol 105:503–511
Stubbs PW, Nielsen JF, Sinkjaer T, Mrachacz-Kersting N (2012) Short-latency crossed spinal responses are impaired differently in sub-acute and chronic stroke patients. Clin Neurophysiol 123:541–549
Acknowledgements
The authors thank Mr. Jan Stavnshøj, and Mr. Knud Larsen for their technical assistance. Grant: This study was funded by a Grant from Det Obelske Familiefond.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mrachacz-Kersting, N., Geertsen, S.S., Stevenson, A.J.T. et al. Convergence of ipsi- and contralateral muscle afferents on common interneurons mediating reciprocal inhibition of ankle plantarflexors in humans. Exp Brain Res 235, 1555–1564 (2017). https://doi.org/10.1007/s00221-016-4871-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-016-4871-6