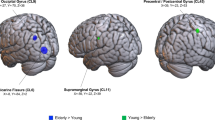
Abstract
Motor imagery (M.I.) is a mental state in which real movements are evoked without overt actions. There is some behavioural evidence that M.I. declines with ageing. The neurofunctional correlates of these changes have been investigated only in two studies, but none of the these studies has measured explicit correlations between behavioural variables and the brain response, nor the correlation of M.I. and motor execution (M.E.) of the same acts in ageing. In this paper, we report a behavioural and functional magnetic resonance imaging (fMRI) experiment that aimed to address this issue. Twenty-four young subjects (27 ± 5.6 years) and twenty-four elderly subjects (60 ± 4.6 years) performed two block-design fMRI tasks requiring actual movement (M.E.) or the mental rehearsal (M.I.) of finger movements. Participants also underwent a behavioural mental chronometry test in which the temporal correlations between M.I. and M.E. were measured. We found significant neurofunctional and behavioural differences between the elderly subjects and the young subjects during the M.E. and the M.I. tasks: for the M.E. task, the elderly subjects showed increased activation in frontal and prefrontal (pre-SMA) cortices as if M.E. had become more cognitively demanding; during the M.I. task, the elderly over-recruited occipito-temporo-parietal areas, suggesting that they may also use a visual imagery strategy. We also found between-group behavioural differences in the mental chronometry task: M.I. and M.E. were highly correlated in the young participants but not in the elderly participants. The temporal discrepancy between M.I. and M.E. in the elderly subjects correlated with the brain regions that showed increased activation in the occipital lobe in the fMRI. The same index was correlated with the premotor regions in the younger subjects. These observations show that healthy elderly individuals have decreased or qualitatively different M.I. compared to younger subjects.






Similar content being viewed by others
Notes
M.I. tasks can be explicit or implicit: in a typical implicit M.I. task, subjects are asked to judge whether a tool is oriented conveniently for being grasped with the right or with the left hand; it is assumed that a mental motor simulation process is used to solve the task. Another example of an implicit M.I. task is the hand laterality judgement task: subjects are asked to judge whether a picture depicts a left rather than a right hand; once debriefed, subjects typically report to have imagined their own hand at the orientation of the visual stimulus. On the other hand, in actual motor tasks, like the one adopted here, subjects are invited to mentally rehearse motor acts as if they were performing them but avoiding overt motor production.
For the finger opposition task, there were minor differences between the fMRI and the task performed outside the scanner (during the behavioural task we varied the number of repetitions of the finger tapping (from 2 to 5 cycle). These were needed in order to collect meaningful behavioural data outside the scanner while keeping the subjects sufficiently involved in the task.
When determining the ideal task for the experiments inside and outside the MRI scanner, a number of factors were taken into account, including compatibility with the fMRI environment and the magnitude of the cortical representation within the motor and premotor cortex for the body segment under investigation. We chose the finger opposition tasks because these tasks have been widely used in functional neuroimaging experiments (see for a review: Witt et al. 2008) and in M.I. investigations using both behavioural (see for example Sirigu et al. 1996) and neurofunctional techniques (see for example Guillot et al. 2009). Based on the same considerations, we decided not to use some interesting motor behaviours, such as pointing (Skoura et al. 2008), lifting one arm (Personnier et al. 2008), or walking (Skoura et al. 2005), despite the potential contribution these tasks could make to the investigation of M.I. in behavioural experiments.
The group by task interaction effects are called “larger activations” or “additional activations,” depending on whether the reference group had rather than not some activations in the given area.
A common objection to the concept of M.I. and its motoric nature as demonstrated by functional neuroimaging is that experimenters may occasionally miss small muscle contractions or even quasi-movements that their volunteers make during tasks. Similar to Jeannerod and Decety (1995), we conceptualise M.I. as a form of cognitive motor rehearsal deprived from an explicit motor outflow. For us, the occasional presence of a green light to spinal motor neurons that manifests itself with occasional motor twitches does not detract from the quality of the mental process under investigation. In addition, the exploration of the neurofunctional activations recorded during M.I. and the direct comparison with the neural activity of the executed motor task reinforces our suggestion. As in previous experiments, we observed commonalities and differences to strongly suggest the following: (1) the likely motoric nature of M.I. given the activation of motor/premotor cortices; (2) the much larger implementation of actual motor acts during M.E. (see the highly significant larger activation of M1/S1 in the M.E. task); and (3) the more cognitive nature of the M.I. task overall, as revealed by the recruitment of higher order premotor and parietal cortices during imagery, particularly in the younger participants.
Our interpretation of the quality of mental imagery in the elderly relies on the distinctive fMRI patterns of the older subjects. We consider these an explicit neural signature because of the topographical distribution in occipital cortices of well-known functional properties. In principle, one could have used introspective descriptions of the M.I. experience to document departures from kinaesthetic imagery to visual imagery and used these departures to decipher the fMRI patterns. However, one may argue that there is no guarantee that introspective descriptions about the accuracy or style of the imagery procedure would be accurate. More crucially, the combination of introspective online descriptions of the quality of the imagery experience during fMRI would have changed the nature of our experiment quite dramatically by turning it into a meta-cognitive protocol about M.I., something very interesting but different from our intended scope. On the other hand, the post hoc correlation of the introspective descriptions with the fMRI activity would have proved temporally inaccurate and possibly difficult to analyse statistically. On the contrary, in our experiment, the emphasis was on explicitly measurable variables, such as the chronometric measures during M.E. and M.I. outside the scanner or the fMRI signal collected during standardised procedures and the ensuing correlations between the two sets of variables.
We cannot claim evidence for a double functional anatomical dissociation, as we did not find significantly greater activations in motor-related structures in the younger subjects. Indeed, while the elderly subjects had greater activations in the occipital cortices, they still relied, in part, on the activity of motor cortices during M.I.
It is worth reemphasising that the elderly also showed activation of the premotor cortices during their M.I. task. Therefore, we suggest that the “visual” mental imagery testified by the occipital additional activation might be a complementary strategy, rather than a completely alternative one.
A similar interpretation was given by Zwergal et al. (2012) in a fMRI study on M.I. during imagined locomotion.
Abbreviations
- BOLD:
-
Blood oxygen level dependent
- FWE:
-
Family-wise error
- fMRI:
-
Functional magnetic resonance imaging
- M.E.:
-
Motor execution
- MEP:
-
Motor-evoked potentials
- M.I.:
-
Motor imagery
- MRI:
-
Magnetic resonance imaging
- SD:
-
Standard deviation
- SMA:
-
Supplementary motor area
- TMS:
-
Transcranial magnetic stimulation
- rTMS:
-
Repetitive transcranial magnetic stimulation
References
Ashburner J, Friston K (1999) Nonlinear spatial normalization using basis functions. Hum Brain Mapp 7(4):254–266
Bartolomeo P (2008) The neural correlates of visual mental imagery: an ongoing debate. Cortex 44(2):107–108
Berlingeri M, Bottini G, Danelli L, Ferri F, Traficante D, Sacheli L, Colombo N, Sberna M, Sterzi R, Scialfa G, Paulesu E (2010) With time on our side? Task-dependent compensatory processes in graceful aging. Exp Brain Res 205(3):307–324
Bonda E, Petrides M, Ostry D, Evans A (1996) Specific involvement of human parietal systems and the amygdala in the perception of biological motion. J Neurosci 16:3737–3744
Buckner RL (2004) Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron 44(1):195–208
Cabeza R (2002) Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging 17(1):85–100
Cabeza R, Anderson ND, Locantore JK, McIntosh AR (2002) Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage 17(3):1394–1402
Calautti C, Serrati C, Baron JC (2001) Effects of age on brain activation during auditory-cued thumb-to-index opposition: a positron emission tomography study. Stroke 32(1):139–146
Carlesimo GA, Buccione I, Fadda L, Graceffa A, Mauri M, Lorusso S, Bevilacqua G et al (2002) Standardizzazione di due test di memoria per uso clinico: Breve Racconto e Figura di Rey. Nuova Riv Neurol 12:1–13
Catalan MJ, Honda M, Weeks RA, Cohen LG, Hallett M (1998) The functional neuroanatomy of simple and complex sequential finger movements: a PET study. Brain 121(2):253–264
Chan RC, Huang J, Di X (2009) Dexterous movement complexity and cerebellar activation: a meta-analysis. Brain Res Rev 59(2):316–323
Coats RO, Wann JP (2011) The reliance on visual feedback control by older adults is highlighted in tasks requiring precise endpoint placement and precision grip. Exp Brain Res 214(1):139–150
Conson M, Sacco S, Sarà M, Pistoia F, Grossi D, Trojano L (2008) Selective motor imagery defect in patients with locked-in syndrome. Neuropsychologia 46(11):2622–2628
Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R (2008) Que PASA? The posterior–anterior shift in aging. Cereb Cortex 18(5):1201–1209
de Lange FP, Hagoort P, Toni I (2005) Neural topography and content of movement representations. J Cogn Neurosci 17(1):97–112
Deblieck C, Pesenti G, Scifo P, Fazio F, Bricolo E, Lo Russo G, Scialfa G, Cossu M, Bottini G, Paulesu E (2003) Preserved functional competence of perilesional areas in drug-resistant epilepsy with lesion in supplementary motor cortex: fMRI and neuropsychological observations. Neuroimage 20(4):2225–2234
Decety J (1996) The neurophysiological basis of motor imagery. Behav Brain Res 77(1–2):45–52
Decety J, Jeannerod M (1995) Mentally simulated movements in virtual reality: does Fitts’s law hold in motor imagery? Behav Brain Res 72(1–2):127–134
Decety J, Jeannerod M, Prablanc C (1989) The timing of mentally represented actions. Behav Brain Res 34(1–2):35–42
Deiber MP, Ibanez V, Honda M, Sadato N, Raman R, Hallett M (1998) Cerebral processes related to visuomotor imagery and generation of simple finger movements studied with positron emission tomography. Neuroimage 7(2):73–85. doi:10.1006/nimg.1997.0314
Dickstein R, Deutsch JE (2007) Motor imagery in physical therapist practice. Phys Ther 87(7):942–953
Folstein MF, Folstein SE, McHugh PR (1975) "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–198
Friston K, Ashburner J, Frith C, Poline J, Heather J, Frackowiak RSJ (1995) Spatial registration and normalization of images. Hum Brain Mapp 2:165–189
Friston K, Holmes A, Price C, Buchel C, Worsley K (1999) Multisubject fMRI studies and conjunction analyses. NeuroImage 10(4):385–396
Ganis G, Thompson WL, Kosslyn SM (2004) Brain areas underlying visual mental imagery and visual perception: an fMRI study. Brain Res Cogn Brain Res 20(2):226–241
Georgopoulos AP, Massey JT (1987) Cognitive spatial-motor processes. 1. The making of movements at various angles from a stimulus direction. Exp Brain Res 65(2):361–370
Gerardin E, Sirigu A, Lehéricy S, Poline JB, Gaymard B, Marsault C, Agid Y, Le Bihan D (2000) Partially overlapping neural networks for real and imagined hand movements. Cereb Cortex 10(11):1093–1104
Giovagnoli AR, Del Pesce M, Mascheroni S, Simoncelli M, Laiacona M, Capitani E (1996) Trail making test: normative values from 287 normal adult controls. Ital J Neurol Sci 17(4):305–309
Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, Pietrini P, Wagner E, Haxby JV (1994) Age-related changes in cortical blood flow activation during visual processing of faces and location. J Neurosci 14(3 Pt 2):1450–1462
Grezes J, Costes N, Decety J (1998) Top-down effect of strategy on the perception of human biological motion: a PET investigation. Cogn Neuropsychol 15:553–582
Guillot A, Collet C (2005) Duration of mentally simulated movement: a review. J Mot Behav 37(1):10–20. doi:10.3200/JMBR.37.1.10-20
Guillot A, Collet C, Nguyen VA, Malouin F, Richards C, Doyon J (2009) Brain activity during visual versus kinesthetic imagery: an fMRI study. Hum Brain Mapp 30(7):2157–2172
Hanakawa T, Immisch I, Toma K, Dimyan M, Van Gelderen P, Hallett M (2003) Functional properties of brain areas associated with motor execution and imagery. J Neurophysiol 89(2):989–1002
Heuninckx S, Wenderoth N, Debaere F, Peeters R, Swinnen SP (2005) Neural basis of aging: the penetration of cognition into action control. J Neurosci 25(29):6787–6796
Hodzic A, Muckli L, Singer W, Stirn A (2009) Cortical responses to self and others. Hum Brain Mapp 30(3):951–962. doi:10.1002/hbm.20558
Holmes A, Friston K (1998) Generalisability, random effects and population inference. NeuroImage 7:S754
Hovington CL, Brouwer B (2010) Guided motor imagery in healthy adults and stroke: does strategy matter? Neurorehabil Neural Repair 24(9):851–857
Hutchinson S, Kobayashi M, Horkan CM, Pascual-Leone A, Alexander MP, Schlaug G (2002) Age-related differences in movement representation. Neuroimage 17(4):1720–1728
Hutsler J, Galuske RA (2003) Hemispheric asymmetries in cerebral cortical networks. Trends Neurosci 26(8):429–435. doi:10.1016/S0166-2236(03)00198-X
Ietswaart M, Johnston M, Dijkerman HC, Joice S, Scott CL, MacWalter RS, Hamilton SJ (2011) Mental practice with motor imagery in stroke recovery: randomized controlled trial of efficacy. Brain 134(Pt 5):1373–1386
Ishai A, Ungerleider LG, Haxby JV (2000) Distributed neural systems for the generation of visual images. Neuron 28(3):979–990
Jackson PL, Doyon J, Richards CL, Malouin F (2004) The efficacy of combined physical and mental practice in the learning of a foot-sequence task after stroke: a case report. Neurorehabil Neural Repair 18(2):106–111
Jeannerod M (2001) Neural simulation of action: a unifying mechanism for motor cognition. Neuroimage 14(1 Pt 2):S103–S109
Jeannerod M, Decety J (1995) Mental motor imagery: a window into the representational stages of action. Curr Opin Neurobiol 5(6):727–732
Jeannerod M, Frak V (1999) Mental imaging of motor activity in humans. Curr Opin Neurobiol 9(6):735–739
Johnson-Frey SH (2004) Stimulation through simulation? Motor imagery and functional reorganization in hemiplegic stroke patients. Brain Cogn 55(2):328–331
Kalisch T, Ragert P, Schwenkreis P, Dinse HR, Tegenthoff M (2009) Impaired tactile acuity in old age is accompanied by enlarged hand representations in somatosensory cortex. Cereb Cortex 19(7):1530–1538
Kauranen K, Vanharanta H (1996) Influences of aging, gender, and handedness on motor performance of upper and lower extremities. Percept Mot Skills 82(2):515–525
Kosslyn SM, Thompson WL, Kim IJ, Alpert NM (1995) Topographical representations of mental images in primary visual cortex. Nature 378(6556):496–498
Kosslyn SM, Pascual-Leone A, Felician O, Camposano S, Keenan JP, Thompson WL, Ganis G, Sukel KE, Alpert NM (1999) The role of area 17 in visual imagery: convergent evidence from PET and rTMS. Science 284(5411):167–170
Lafleur MF, Jackson PL, Malouin F, Richards CL, Evans AC, Doyon J (2002) Motor learning produces parallel dynamic functional changes during the execution and imagination of sequential foot movements. Neuroimage 16(1):142–157. doi:10.1006/nimg.2001.1048
Lau HC, Rogers RD, Haggard P, Passingham RE (2004) Attention to intention. Science 303(5661):1208–1210
Lenz M, Tegenthoff M, Kohlhaas K, Stude P, Hoffken O, Gatica Tossi MA, Kalisch T, Dinse HR (2012) Increased excitability of somatosensory cortex in aged humans is associated with impaired tactile acuity. J Neurosci 32(5):1811–1816
Leonard G, Tremblay F (2007) Corticomotor facilitation associated with observation, imagery and imitation of hand actions: a comparative study in young and old adults. Exp Brain Res 177(2):167–175
Liu KP, Chan CC, Lee TM, Hui-Chan CW (2004) Mental imagery for relearning of people after brain injury. Brain Inj 18(11):1163–1172
Lotze M, Cohen LG (2006) Volition and imagery in neurorehabilitation. Cogn Behav Neurol 19(3):135–140
Lulé D, Diekmann V, Kassubek J, Kurt A, Birbaumer N, Ludolph AC, Kraft E (2007) Cortical plasticity in amyotrophic lateral sclerosis: motor imagery and function. Neurorehabil Neural Repair 21(6):518–526
Lumer ED, Friston KJ, Rees G (1998) Neural correlates of perceptual rivalry in the human brain. Science 280(5371):1930–1934
Malouin F, Richards CL, Doyon J, Desrosiers J, Belleville S (2004) Training mobility tasks after stroke with combined mental and physical practice: a feasibility study. Neurorehabil Neural Repair 18(2):66–75
Malouin F, Richards CL, Durand A (2010) Normal aging and motor imagery vividness: implications for mental practice training in rehabilitation. Arch Phys Med Rehabil 91(7):1122–1127
Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, Weinberger DR (2002) Neurophysiological correlates of age-related changes in human motor function. Neurology 58(4):630–635
Mellet E, Petit L, Mazoyer B, Denis M, Tzourio N (1998) Reopening the mental imagery debate: lessons from functional anatomy. Neuroimage 8(2):129–139
Mendola JD, Dale AM, Fischl B, Liu AK, Tootell RB (1999) The representation of illusory and real contours in human cortical visual areas revealed by functional magnetic resonance imaging. J Neurosci 19(19):8560–8572
Mulder T (2007) Motor imagery and action observation: cognitive tools for rehabilitation. J Neural Transm 114(10):1265–1278
Mulder T, Hochstenbach JB, van Heuvelen MJ, den Otter AR (2007) Motor imagery: the relation between age and imagery capacity. Hum Mov Sci 26(2):203–211
Nachev P, Kennard C, Husain M (2008) Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci 9(11):856–869
Nedelko V, Hassa T, Hamzei F, Weiller C, Binkofski F, Schoenfeld MA, Tüscher O, Dettmers C (2010) Age-independent activation in areas of the mirror neuron system during action observation and action imagery. A fMRI study. Restor Neurol Neurosci 28(6):737–747
Novelli G, Papagno C, Capitani E, Laiacona M, Vallar G, Cappa SF (1986) Three clinical tests for the assessment of verbal long-term memory function: norms from 320 normal subjects. Arch Psicol Neurol Psichiatr 47:278–296
O’Craven KM, Kanwisher N (2000) Mental imagery of faces and places activates corresponding stiimulus-specific brain regions. J Cogn Neurosci 12(6):1013–1023
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9(1):97–113
Orsini A, Grossi D, Capitani E, Laiacona M, Papagno C, Vallar G (1987) Verbal and spatial immediate memory span: normative data from 1355 adults and 1112 children. Ital J Neurol Sci 8(6):539–548
Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD (2006) Detecting awareness in the vegetative state. Science 313(5792):1402
Page SJ, Levine P, Sisto S, Johnston MV (2001) A randomized efficacy and feasibility study of imagery in acute stroke. Clin Rehabil 15(3):233–240
Park DC, Reuter-Lorenz P (2009) The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol 60:173–196
Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M (1995) Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol 74(3):1037–1045
Penny W, Holmes AP (2004) Random-effects analysis. In: Frackowiak RJS et al. (eds) Human brain function. Elsevier, San Diego, pp 843–850
Personnier P, Paizis C, Ballay Y, Papaxanthis C (2008) Mentally represented motor actions in normal aging II. The influence of the gravito-inertial context on the duration of overt and covert arm movements. Behav Brain Res 186(2):273–283
Personnier P, Ballay Y, Papaxanthis C (2010a) Mentally represented motor actions in normal aging: III. Electromyographic features of imagined arm movements. Behav Brain Res 206(2):184–191
Personnier P, Kubicki A, Laroche D, Papaxanthis C (2010b) Temporal features of imagined locomotion in normal aging. Neurosci Lett 476(3):146–149
Phothisonothai M, Nakagawa M (2009) A classification method of different motor imagery tasks based on fractal features for brain-machine interface. J Integr Neurosci 8(1):95–122
Raven J (1984) CPM. Coloured Progressive Matrices. OS, Firenze
Rizzolatti G, Craighero L (2004) The mirror-neuron system. Annu Rev Neurosci 27:169–192
Saimpont A, Pozzo T, Papaxanthis C (2009) Aging affects the mental rotation of left and right hands. PLoS One 4(8):e6714
Saxe R, Jamal N, Powell L (2006) My body or yours? The effect of visual perspective on cortical body representations. Cereb Cortex 16(2):178–182. doi:10.1093/cercor/bhi095
Seghier ML (2008) Laterality index in functional MRI: methodological issues. Magn Reson Imaging 26(5):594–601. doi:10.1016/j.mri.2007.10.010
Sereno MI, McDonald CT, Allman JM (1994) Analysis of retinotopic maps in extrastriate cortex. Cereb Cortex 4(6):601–620
Sirigu A, Duhamel JR, Cohen L, Pillon B, Dubois B, Agid Y (1996) The mental representation of hand movements after parietal cortex damage. Science 273(5281):1564–1568
Skoura X, Papaxanthis C, Vinter A, Pozzo T (2005) Mentally represented motor actions in normal aging. I. Age effects on the temporal features of overt and covert execution of actions. Behav Brain Res 165(2):229–239
Skoura X, Personnier P, Vinter A, Pozzo T, Papaxanthis C (2008) Decline in motor prediction in elderly subjects: right versus left arm differences in mentally simulated motor actions. Cortex 44(9):1271–1278
Smith CD, Umberger GH, Manning EL, Slevin JT, Wekstein DR, Schmitt FA, Markesbery WR, Zhang Z, Gerhardt GA, Kryscio RJ, Gash DM (1999) Critical decline in fine motor hand movements in human aging. Neurology 53(7):1458–1461
Spinnler H, Tognoni G (1987) Standardizzazione e taratura italiana di test neuropsicologici. Masson Italia Periodici, Milano
Stenekes MW, Geertzen JH, Nicolai JP, De Jong BM, Mulder T (2009) Effects of motor imagery on hand function during immobilization after flexor tendon repair. Arch Phys Med Rehabil 90(4):553–559
Stephan KM, Fink GR, Passingham RE, Silbersweig D, Ceballos-Baumann AO, Frith CD, Frackowiak RS (1995) Functional anatomy of the mental representation of upper extremity movements in healthy subjects. J Neurophysiol 73(1):373–386
Stevens JA (2005) Interference effects demonstrate distinct roles for visual and motor imagery during the mental representation of human action. Cognition 95(3):329–350
Strauss E, Kosaka B, Wada J (1983) The neurobiological basis of lateralized cerebral function. A review. Hum Neurobiol 2(3):115–127
Vogt S (1995) On relations between perceiving, imagining and performing in the learning of cyclical movement sequences. Br J Psychol 86(Pt 2):191–216
Ward NS, Frackowiak RS (2003) Age-related changes in the neural correlates of motor performance. Brain 126(Pt 4):873–888
Wechsler D (1945) A standardized memory scale for clinical use. J Psychol 19:87–95
Witt ST, Laird AR, Meyerand ME (2008) Functional neuroimaging correlates of finger-tapping task variations: an ALE meta-analysis. Neuroimage 42(1):343–356
Worsley K, Friston K (1995) Analysis of fMRI time-series revisited—again. NeuroImage 2:173–181
Worsley K, Friston K (2000) A test for a conjunction. Stat Probab Lett 47:135–140
Worsley K, Marrett S, Neelin P, Vandal A, Friston K, Evans A (1996) A unified statistical approach for determining significant voxels in images of cerebral activation. Hum Brain Mapp 4:58–73
Wu T, Hallett M (2005) The influence of normal human ageing on automatic movements. J Physiol 562(Pt 2):605–615
Yue G, Cole KJ (1992) Strength increases from the motor program: comparison of training with maximal voluntary and imagined muscle contractions. J Neurophysiol 67(5):1114–1123
Zeki S, Watson JD, Lueck CJ, Friston KJ, Kennard C, Frackowiak RS (1991) A direct demonstration of functional specialization in human visual cortex. J Neurosci 11(3):641–649
Zwergal A, Linn J, Xiong G, Brandt T, Strupp M, Jahn K (2012) Aging of human supraspinal locomotor and postural control in fMRI. Neurobiol Aging 33(6):1073–1084
Acknowledgments
We thank the staff of the Department of Diagnostic Radiology and Bioimages of IRCCS Galeazzi and the Department of Neuroradiology of Niguarda Hospital.
Author information
Authors and Affiliations
Corresponding author
Additional information
L. Zapparoli and P. Invernizzi contributed equally to the authorship of this paper.
Rights and permissions
About this article
Cite this article
Zapparoli, L., Invernizzi, P., Gandola, M. et al. Mental images across the adult lifespan: a behavioural and fMRI investigation of motor execution and motor imagery. Exp Brain Res 224, 519–540 (2013). https://doi.org/10.1007/s00221-012-3331-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-012-3331-1