Abstract
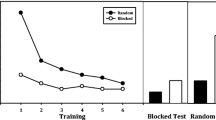
The dorsal premotor cortex (PMd) and the supplementary motor area (SMA) are critical for the acquisition and expression of sequential behavior, but little is known regarding how these regions are recruited when we must simultaneously acquire multiple sequences under different amounts of training. We hypothesized that these regions contribute to the retrieval of sequences at different familiarity levels, with the left PMd supporting sequences of moderate familiarity and the SMA supporting sequences of greater familiarity. Double-pulse transcranial magnetic stimulation (TMS) was applied during the retrieval of six sequences previously learned under three different amounts of exposure during 30 days of training using a discrete sequence production task. TMS led to a significant interaction of sequence error between depth of training and stimulation location. Stimulation of the left PMd increased error during moderate sequence retrieval, whereas stimulation of the SMA increased error during the retrieval of both moderately and extensively trained sequences. The lack of a double dissociation fails to support a direct correspondence between brain region and putative behavioral learning stage. Instead, the interaction suggests that SMA and PMd support the expression of sequences over different, albeit overlapping, time scales. Separate analysis of sequence initiation time did not demonstrate any significant difference between moderately and extensively trained sequences. Instead, stimulation to either region quickened sequence initiation for these sequences, but not for those sequences with poor retrieval performance. This supports the general role of these premotor regions in the maintenance of specific sequence knowledge prior to movement onset.




Similar content being viewed by others
References
Bischoff-Grethe A, Goedert KM, Willingham DT, Grafton ST (2004) Neural substrates of response-based sequence learning using fMRI. J Cogn Neurosci 16:127–138
Cross ES, Schmitt PJ, Grafton ST (2007) Neural substrates of contextual interference during motor learning support a model of active preparation. J Cogn Neurosci 19:1854–1871
Dayan E, Cohen LG (2011) Neuroplasticity subserving motor skill learning. Neuron 72:443–454
Deiber MP, Wise SP, Honda M, Catalan MJ, Grafman J, Hallett M (1997) Frontal and parietal networks for conditional motor learning: a positron emission tomography study. J Neurophysiol 78:977–991
Doyon J, Song AW, Karni A, Lalonde F, Adams MM, Ungerleider LG (2002) Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc Natl Acad Sci USA 99:1017–1022
Duque J, Ivry RB (2009) Role of corticospinal suppression during motor preparation. Cereb Cortex 19:2013–2024
Duque J, Labruna L, Verset S, Olivier E, Ivry RB (2012) Dissociating the role of prefrontal and premotor cortices in controlling inhibitory mechanisms during motor preparation. J Neurosci 32:806–816
Gerloff C, Corwell B, Chen R, Hallett M, Cohen LG (1997) Stimulation over the human supplementary motor area interferes with the organization of future elements in complex motor sequences. Brain 120:1587–1602
Ghilardi MF, Moisello C, Silvestri G, Ghez C, Krakauer JW (2009) Learning of a sequential motor skill comprises explicit and implicit components that consolidate differently. J Neurophysiol 101:2218–2229
Goedert KM, Willingham DB (2002) Patterns of interference in sequence learning and prism adaptation inconsistent with the consolidation hypothesis. Learn Mem 9:279–292
Grafton ST, Hazeltine E, Ivry R (1995) Functional mapping of sequence learning in normal humans. J Cogn Neurosci 7:497–510
Grafton ST, Hazeltine E, Ivry RB (1998) Abstract and effector-specific representations of motor sequences identified with PET. J Neurosci 18:9420–9428
Haaland KY, Elsinger CL, Mayer AR, Durgerian S, Rao SM (2004) Motor sequence complexity and performing hand produce differential patterns of hemispheric lateralization. J Cogn Neurosci 16:621–636
Hamada M, Hanajima R, Terao Y, Okabe S, Nakatani-Enomoto S, Furubayashi T, Matsumoto H, Shirota Y, Ohminami S, Ugawa Y (2009) Primary motor cortical metaplasticity induced by priming over the supplementary motor area. J Physiol 587:4845–4862
Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ (1995) Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain 118:913–933
Jenkins IH, Brooks DJ, Nixon PD, Frackowiak RSJ, Passingham RS (1994) Motor sequence learning: a study with positron emission tomography. J Neurosci 14:3775–3790
Jenkins IH, Jahanshahi M, Jueptner M, Passingham RE, Brooks DJ (2000) Self-initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow. Brain 123:1216–1228
Jueptner M, Stephan KM, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE (1997) Anatomy of motor learning. I. Frontal cortex and attention to action. J Neurophysiol 77:1313–1324
Koch G, Franca M, Mochizuki H, Marconi B, Caltagirone C, Rothwell JC (2007) Interactions between pairs of transcranial magnetic stimuli over the human left dorsal premotor cortex differ from those seen in primary motor cortex. J Physiol 578:551–562
Krakauer JW, Ghez C, Ghilardi MF (2005) Adaptation to visuomotor transformations: consolidation, interference, and forgetting. J Neurosci 25:473–478
Lehéricy S, Benali H, Van de Moortele P-F, Pélégrini-Issac M, Waechter T, Ugurbil K, Doyon J (2005) Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci USA 102:12566–12571
Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M (2000) Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain 123:1161–1173
Matsuzaka Y, Picard N, Strick PL (2007) Skill representation in the primary motor cortex after long-term practice. J Neurophysiol 97:1819–1832
Mushiake H, Inase M, Tanji J (1991) Neuronal activity in the primate premotor, supplementary, and precentral motor cortex during visually guided and internally determined sequential movements. J Neurophysiol 66:705–718
Osu R, Hirai S, Yoshioka T, Kawato M (2004) Random presentation enables subjects to adapt to two opposing forces on the hand. Nat Neurosci 7:111–112
Picard N, Strick PL (2001) Imaging the premotor areas. Curr Opin Neurobiol 11:663–672
Rhodes BJ, Bullock D, Verwey WB, Averbeck BB, Page MPA (2004) Learning and production of movement sequences: behavioral, neurophysiological, and modeling perspectives. Hum Mov Sci 23:699–746
Shea JB, Morgan RL (1979) Contextual interference effects on the acquisition, retention, and transfer of a motor skill. J Exp Psychol Hum Learn Mem 5:179–187
Stinear CM, Barber PA, Coxon JP, Verryt TS, Acharya PP, Byblow WD (2009) Repetitive stimulation of premotor cortex affects primary motor cortex excitability and movement preparation. Brain Stimul 2:152–162
Tanji J (2001) Sequential organization of multiple movements: involvement of cortical motor areas. Annu Rev Neurosci 24:631–651
Verstynen T, Diedrichsen J, Albert N, Aparicio P, Ivry RB (2005) Ipsilateral motor cortex activity during unimanual hand movements relates to task complexity. J Neurophysiol 93:1209–1222
Verwey WB, Lammens R, van Honk J (2002) On the role of the SMA in the discrete sequence production task: a TMS study. Neuropsychologia 40:1268–1276
Walker MP, Brakefield T, Hobson JA, Stickgold R (2003) Dissociable stages of human memory consolidation and reconsolidation. Nature 425:616–620
Wise SP (1985) The primate premotor cortex: past, present, and preparatory. Annu Rev Neurosci 8:1–19
Acknowledgments
This study was supported by Public Health Service grant NS44393 and the Institute for Collaborative Biotechnologies through Contract W911NF-09-D-0001 from the US Army Research Office, and the National Science Foundation (DMS-0645369).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wymbs, N.F., Grafton, S.T. Contributions from the left PMd and the SMA during sequence retrieval as determined by depth of training. Exp Brain Res 224, 49–58 (2013). https://doi.org/10.1007/s00221-012-3287-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-012-3287-1