Abstract
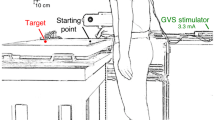
A general theme in sensory perception is that exposure to a stimulus makes it seem more neutral such that perception of subsequent stimuli is shifted in the opposite direction. The visual motion aftereffect (MAE) is an extensively studied example of this. Although similar effects have been described in other sensory systems, it has not previously been described in the vestibular system. Velocity storage has been extensively studied in the vestibular system and suggests a persistence of perception in the direction of the initial movement. The current study sought to determine how motion perception is influenced by prior movement in darkness. Thirteen human subjects (mean age 41, range 21–68) underwent whole-body fore–aft translation. The threshold of vestibular motion discrimination perception was measured using a single interval (1I) of motion lasting 0.5 s in which subjects identified their direction of motion as forward or backward using an adaptive staircase. The translation aftereffect (TAE) was measured in 2-interval (2I) experiments: The adapting stimulus moved 15 cm in 1.5 s (peak velocity 20 cm/s, peak acceleration 42 cm/s2). After a fixed inter-stimulus interval (ISI) of 0.5, 1.0, 1.5, or 3 s, a second stimulus lasting 0.5 s was delivered and the subject identified the perceived direction of the second test stimulus. The test stimulus was determined using an adaptive staircase. The ISI was constant within the block, but adapting stimuli directions were randomly interleaved. During the 1I condition, the response bias was near zero in all subjects. With a 2I stimulus, 8 of 13 subjects demonstrated a significant bias. At an ISI of 0.5 s, a minority of subjects demonstrated a bias in the same direction as the adapter. When the ISI was 1, 1.5, or 3 s, all subjects who demonstrated a significant TAE had one in the opposite direction of the adapter, similar to that seen for MAE. When averaged across subjects, the TAE was significant with ISIs of 1.0 s and above. These findings demonstrate that perception of vestibular stimuli depends on prior motion. This has important implications for understanding and quantifying vestibular perception.






Similar content being viewed by others
References
Addams R (1834) An account of a peculiar optical phenomenon seen after having looked at a moving body etc. Mag J Sci 5:373–374 (3rd series)
Andersen GJ, Braunstein ML (1985) Induced self-motion in central vision. J Exp Psychol Hum Percept Perform 11:122–132
Angelaki DE, Dickman JD (2000) Spatiotemporal processing of linear acceleration: primary afferent and central vestibular neuron responses. J Neurophysiol 84:2113–2132
Angelaki DE, Gu Y, Deangelis GC (2009) Multisensory integration: psychophysics, neurophysiology, and computation. Curr Opin Neurobiol 19:1–7
Anstis S, Verstraten FA, Mather G (1998) The motion aftereffect. Trends Cogn Sci 2:111–117
Barlow HB (1990) A theory about the functional role and synaptic mechanism of visual aftereffects. In: Blakemore C (ed) Vision: coding and efficiency. Cambridge University Press, Cambridge, pp 363–375
Barnett-Cowan M, Harris LR (2009) Perceived timing of vestibular stimulation relative to touch, light and sound. Exp Brain Res 198:221–231. doi:10.1007/s00221-009-1779-4
Benson AJ, Spencer MB, Stott JR (1986) Thresholds for the detection of the direction of whole-body, linear movement in the horizontal plane. Aviat Space Environ Med 57:1088–1096
Benson AJ, Hutt EC, Brown SF (1989) Thresholds for the perception of whole body angular movement about a vertical axis. Aviat Space Environ Med 60:205–213
Bertolini G, Bockisch CJ, Straumann D, Zee DS, Ramat S (2008) Do humans show velocity-storage in the vertical rVOR? Prog Brain Res 171:207–210. doi:10.1016/S0079-6123(08)00628-6
Bertolini G, Ramat S, Laurens J, Bockisch CJ, Marti S, Straumann D, Palla A (2011) Velocity storage contribution to vestibular self-motion perception in healthy human subjects. J Neurophysiol 105:209–223. doi:10.1152/jn.00154.2010
Bestelmeyer PE, Rouger J, DeBruine LM, Belin P (2010) Auditory adaptation in vocal affect perception. Cognition 117:217–223. doi:10.1016/j.cognition.2010.08.008
Clement G, Moore ST, Raphan T, Cohen B (2001) Perception of tilt (somatogravic illusion) in response to sustained linear acceleration during space flight. Exp Brain Res 138:410–418
Cohen H, Cohen B, Raphan T, Waespe W (1992) Habituation and adaptation of the vestibuloocular reflex: a model of differential control by the vestibulocerebellum. Exp Brain Res 90:526–538
Crane BT, Demer JL (2000) Effect of adaptation to telescopic spectacles on the initial human horizontal vestibuloocular reflex. J Neurophysiol 83:38–49
Eatock RA (2000) Adaptation in hair cells. Annu Rev Neurosci 23:285–314. doi:10.1146/annurev.neuro.23.1.285
Ercoline WR, Devilbiss CA, Yauch DW, Brown DL (2000) Post-roll effects on attitude perception: “the Gillingham Illusion”. Aviat Space Environ Med 71:489–495
Fernandez C, Goldberg JM (1976) Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. I. Response to static tilts and to long-duration centrifugal force. J Neurophysiol 39:970–984
Fetsch CR, Turner AH, Deangelis GC, Angelaki DE (2009) Dynamic re-weighting of visual and vestibular cues during self-motion perception. J Neurosci 29:15601–15612
Gescheider GA, Wright JH (1969) Effects of vibrotactile adaptation on the perception of stimuli of varied intensity. J Exp Psychol 81:449–453
Gianna CC, Heimbrand S, Nakamura T, Gresty MA (1995) Thresholds for perception of lateral motion in normal subjects and patients with bilateral loss of vestibular function. Acta Otolaryngol Suppl 520(Pt 2):343–346
Gibson JJ (1950) The perception of the visual world. Houghton Mifflin, Boston
Glasser DM, Tsui JM, Pack CC, Tadin D (2011) Perceptual and neural consequences of rapid motion adaptation. In: Proceedings of the National Academy of Sciences of the United States of America. doi:10.1073/pnas.1101141108
Grabherr L, Nicoucar K, Mast FW, Merfeld DM (2008) Vestibular thresholds for yaw rotation about an earth-vertical axis as a function of frequency. Exp Brain Res 186:677–681
Gu Y, Fetsch CR, Adeyemo B, Deangelis GC, Angelaki DE (2010) Decoding of MSTd population activity accounts for variations in the precision of heading perception. Neuron 66:596–609. doi:10.1016/j.neuron.2010.04.026
Harris LR, Morgan MJ, Still AW (1981) Moving and the motion after-effect. Nature 293:139–141
Hershenson M (1989) Duration, time constant, and decay of the linear motion aftereffect as a function of inspection duration. Percept Psychophys 45:251–257
Hess BJ, Angelaki DE (1997) Inertial vestibular coding of motion: concepts and evidence. Curr Opin Neurobiol 7:860–866
Johansson G (1977) Studies on visual perception of locomotion. Perception 6:365–376
Kanai R, Verstraten FA (2005) Perceptual manifestations of fast neural plasticity: motion priming, rapid motion aftereffect and perceptual sensitization. Vision Res 45:3109–3116. doi:10.1016/j.visres.2005.05.014
Lundstrom RJ (1986) Responses of mechanoreceptive afferent units in the glabrous skin of the human hand to vibration. Scand J Work Environ Health 12:413–416
Lyons TJ, Ercoline WR, Freeman JE, Gillingham KK (1994) Classification problems of U.S. Air Force spatial disorientation accidents, 1989–9191. Aviat Space Environ Med 65:147–152
MacNeilage PR, Banks MS, DeAngelis GC, Angelaki DE (2010) Vestibular heading discrimination and sensitivity to linear acceleration in head and world coordinates. J Neurosci 30:9084–9094. doi:10.1523/JNEUROSCI.1304-10.2010
Merfeld DM, Gong W, Morrissey J, Saginaw M, Haburcakova C, Lewis RF (2006) Acclimation to chronic constant-rate peripheral stimulation provided by a vestibular prosthesis. IEEE Trans Biomed Eng 53:2362–2372. doi:10.1109/TBME.2006.883645
Miles FA, Eighmy BB (1980) Long-term adaptive changes in primate vestibuloocular reflex. I. Behavioral observations. J Neurophysiol 43:1406–1425
Nooij SA, Groen EL (2011) Rolling into spatial disorientation: simulator demonstration of the post-roll (Gillingham) illusion. Aviat Space Environ Med 82:505–512
Ohmi M, Howard IP (1988) Effect of stationary objects on illusory forward self-motion induced by a looming display. Perception 17:5–11
Paige GD, Tomko DL (1991) Eye movement responses to linear head motion in the squirrel monkey. I. Basic characteristics. J Neurophysiol 65:1170–1182
Pinkus A, Pantle A (1997) Probing visual motion signals with a priming paradigm. Vision Res 37:541–552
Raphan T, Matsuo V, Cohen B (1979) Velocity storage in the vestibulo-ocular reflex arc (VOR). Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale 35:229–248
Reinhardt-Rutland AH (1998) Increasing-loudness aftereffect following decreasing-intensity adaptation: spectral dependence in interotic and monotic testing. Perception 27:473–482
Roditi RE, Crane BT (2012) Directional asymmetries and age effects in human self-motion perception. J Assoc Res Otolaryngol [Epub ahead of print]
Seizova-Cajic T, Smith JL, Taylor JL, Gandevia SC (2007) Proprioceptive movement illusions due to prolonged stimulation: reversals and aftereffects. PLoS ONE 2:e1037. doi:10.1371/journal.pone.0001037
Seno T, Ito H, Sunaga S (2010) Vection aftereffects from expanding/contracting stimuli. Seeing perceiving 23:273–294
Soyka F, Robuffo Giordano P, Beykirch K, Bulthoff HH (2011) Predicting direction detection thresholds for arbitrary translational acceleration profiles in the horizontal plane. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale 209:95–107. doi:10.1007/s00221-010-2523-9
Thompson P, Burr D (2009) Visual aftereffects. Current biology: CB 19:R11–R14. doi:10.1016/j.cub.2008.10.014
Tomlinson RD, McConville KM, Na EQ (1996) Behavior of cells without eye movement sensitivity in the vestibular nuclei during combined rotational and translational stimuli. J Vestib Res 6:145–158
Ullman S, Schechtman G (1982) Adaptation and gain normalization. Proceedings of the Royal Society of London. Series B, Containing papers of a biological character. Royal Soc 216:299–313
Wade NJ (1994) A selective history of the study of visual motion aftereffects. Perception 23:1111–1134
Wallach H, Flaherty EW (1975) A compensation for field expansion caused by moving forward. Percept Psychophys 17:445–449
Walsh EG (1961) Role of the vestibular apparatus in the perception of motion on a parallel swing. J Physiol 155:506–513
Wang Q, Webber RM, Stanley GB (2010) Thalamic synchrony and the adaptive gating of information flow to cortex. Nat Neurosci 13:1534–1541. doi:10.1038/nn.2670
Wichmann FA, Hill NJ (2001a) The psychometric function: I. Fitting, sampling, and goodness of fit. Percept Psychophys 63:1293–1313
Wichmann FA, Hill NJ (2001b) The psychometric function: II. Bootstrap-based confidence intervals and sampling. Percept Psychophys 63:1314–1329
Young LR, Oman CM (1969) Model for vestibular adaptation to horizontal rotation. Aerosp Med 40:1076–1080
Acknowledgments
This work was funded by a grant from the National Institute on Deafness and Other Communication Disorders K23 DC011298. Additional support was provided by the American Otological Society and a grant from the Triological Society. Technical support was provided by Shawn Olmstead-Leahey.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Crane, B.T. Fore–aft translation aftereffects. Exp Brain Res 219, 477–487 (2012). https://doi.org/10.1007/s00221-012-3105-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-012-3105-9