Abstract
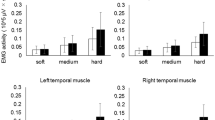
Using functional magnetic resonance imaging (fMRI) and near-infrared spectroscopy (NIRS), we examined the role of periodontal afferent inputs on cerebral activation pattern evoked by masticatory muscle activity in twenty-two subjects. Statistical comparisons were used to identify brain regions with significant activation after subtraction of baseline activity from sham teeth-tapping (no periodontal input) and teeth-tapping (periodontal input) activity in an fMRI (N = 14) and NIRS study (N = 8). Both sham teeth-tapping and teeth-tapping significantly activated bilateral sensorimotor cortex and supplementary motor area in the fMRI study. NIRS revealed that oxygenated hemoglobin concentrations increased in sensorimotor cortex; however, there was no significant difference in degree of oxygenated hemoglobin changes between sham teeth-tapping and teeth-tapping. A control study (N = 8) characterized the jaw muscle activity and amplitude of the two motor tasks and demonstrated significantly higher electromyogram (EMG) activity in the jaw closing muscles during teeth contact in the teeth-tapping session. Since the cerebral activation during sham teeth-tapping and teeth-tapping was similar, we suggest that the influence of periodontal afferent inputs and associated jaw muscle activity is relatively minor compared to the rhythmic jaw movements. Although the clinical significance of the present findings remains unknown, they may have implications for the understanding of awake or sleep-related bruxism characterized by subconscious and rhythmic teeth-grinding or teeth-clenching.




Similar content being viewed by others
References
Baad-Hansen L, Blicher JU, Lapitskaya N, Nielsen JF, Svensson P (2009) Intra-cortical excitability in healthy human subjects after tongue training. J Oral Rehabil 36:427–434
Benaron DA, Hintz SR, Villringer A, Boas D, Kleinschmidt A, Frahm J, Hirth C, Obrig H, van Houten JC, Kermit EL, Cheong WF, Stevenson DK (2000) Noninvasive functional imaging of human brain using light. J Cereb Blood Flow Metab 20:469–477
Byrd KE, Romito LM, Dzemidzic M, Wong D, Talavage TM (2009) fMRI study of brain activity elicited by oral parafunctional movements. J Oral Rehabil 36:346–361
Cannestra AF, Pouratian N, Bookheimer SY, Martin NA, Beckerand DP, Toga AW (2001) Temporal spatial differences observed by functional MRI and human intraoperative optical imaging. Cereb Cortex 11:773–782
Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ (1994) Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2:189–210
Fujiwara N, Sakatani K, Katayama Y, Murata Y, Hoshino T, Fukaya C, Yamamoto T (2004) Evoked-cerebral blood oxygenation changes in false-negative activations in BOLD contrast functional MRI of patients with brain tumors. Neuroimage 21:1464–1471
Ganesh G, Burdet E, Haruno M, Kawato M (2008) Sparse linear regression for reconstructing muscle activity from human cortical fMRI. Neuroimage 42:1463–1472
Hasegawa Y, Ono T, Hori K, Nokubi T (2007) Influence of human jaw movement on cerebral blood flow. J Dent Res 86:64–68
Hoshi Y, Kobayashi N, Tamura M (2001) Interpretation of near-infrared spectroscopy signals: a study with a newly developed perfused rat brain model. J Appl Physiol 90:1657–1662
Iida T, Fenwick PB, Ioannides AA (2007) Analysis of brain activity immediately before conscious teeth clenching using magneto encephalographic method. J Oral Rehabil 34:487–496
Iida T, Kato M, Komiyama O, Suzuki H, Asano T, Kuroki T, Kaneda T, Svensson P, Kawara M (2010a) Comparison of cerebral activity during teeth clenching and fist clenching: a functional magnetic resonance imaging study. Eur J Oral Sci 118:635–641
Iida T, Kawara M, Hironaga N, Ioannides AA (2010b) Cerebellar activity before teeth-clenching using magnetoencephalography. J Prosthodont Res 54:48–52
Isobe K, Kusaka T, Nagano K, Okubo K, Yasuda S, Kondo M, Itoh S, Onishi S (2001) Functional imaging of the brain in sedated newborn infants using near infrared topography during passive knee movement. Neurosci Lett 299:221–224
Jiang H, Liu H, Liu G, Jin Z, Liu X (2010) The effects of chewing-side preference on human brain activity during tooth clenching: an fMRI study. J Oral Rehabil 37:877–883
Kimoto K, Ono Y, Tachibana A, Hirano Y, Otsuka T, Ohno A, Yamaya K, Obata T, Onozuka M (2011) Chewing-induced regional brain activity in edentulous patients who received mandibular implant-supported overdentures: a preliminary report. J Prosthodont Res 55(2):89–97
Kleinschmidt A, Obrig H, Requardt M, Merboldt KD, Dirnagl U, Villringer A, Frahm J (1996) Simultaneous recording of cerebral blood oxygenation changes during human brain activation by magnetic resonance imaging and near-infrared spectroscopy. J Cereb Blood Flow Metab 165:817–826
Manganotti P, Acler M, Formaggio E, Avesani M, Milanese F, Baraldo A, Storti SF, Gasparini A, Cerini R, Mucelli RP, Fiaschi A (2010) Changes in cerebral activity after decreased upper-limb hypertonus: an EMG-fMRI study. Magn Reson Imaging 28:646–652
Miyai I, Tanabe HC, Sase I, Eda H, Oda I, Konishi I, Tsunazawa Y, Suzuki T, Yanagida T, Kubota K (2001) Cortical mapping of gait in humans: a near-infrared spectroscopic topography study. Neuroimage 14:1186–1192
Momose I, Nishikawa J, Watanabe T, Sasaki Y, Senda M, Kubota K, Sato Y, Funakoshi M, Minakuchi S (1997) Effect of mastication on regional cerebral blood flow in humans examined by positron-emission tomography with 15O-labelled water and magnetic resonance imaging. Arch Oral Biol 42:57–61
Murata Y, Sakatani K, Katayama Y, Fukaya C (2002) Increase in focal concentration of deoxyhemoglobin during neuronal activity in cerebral ischemic patients. J Neurol Neurosurg Psychiatr 73:182–184
Narita N, Kamiya K, Yamamura K, Kawasaki S, Matsumoto T, Tanaka N (2009) Chewing-related prefrontal cortex activation while wearing partial denture prosthesis: pilot study. J Prosthodont Res 53:126–135
Noguchi Y, Takeuchi T, Sakai KL (2002) Lateralized activation in the inferior frontal cortex during syntactic processing: event-related optical topography study. Hum Brain Mapp 17:89–99
Okamoto M, Dan H, Shimizu K, Takeo K, Amita T, Oda I, Konishi I, Sakamoto K, Isobe S, Suzuki T, Kohyama K, Dan I (2004) Multimodal assessment of cortical activation during apple peeling by NIRS and fMRI. Neuroimage 21:1275–1288
Onozuka M, Fujita M, Watanabe K, Hirano Y, Niwa M, Nishiyama K, Saito S (2002) Mapping brain region activity during chewing: a functional magnetic resonance imaging study. J Dent Res 81:743–746
Penfield W, Boldrey E (1937) Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60:389–443
Sakatani K, Murata Y, Fujiwara N, Hoshino T, Nakamura S, Kano T, Katayama Y (2007) Comparison of blood-oxygen-level-dependent functional magnetic resonance imaging and near-infrared spectroscopy recording during functional brain activation in patients with stroke and brain tumors. J Biomed Opt 12:062110
Shibusawa M, Takeda T, Nakajima K, Ishigami K, Sakatani K (2009) Functional near-infrared spectroscopy study on primary motor and sensory cortex response to clenching. Neurosci Lett 449:98–102
Svensson P, Arendt-Nielsen L, Houe L (1998) Muscle pain modulates mastication: an experimental study in humans. J Orofac Pain 12(1):7–16
Svensson P, Romaniello A, Arendt-Nielsen L, Sessle BJ (2003) Plasticity in corticomotor control of the human tongue musculature induced by tongue-task training. Exp Brain Res 152:42–51
Svensson P, Romaniello A, Wang K, Arendt-Nielsen L, Sessle BJ (2006) One hour of tongue-task training is associated with plasticity in corticomotor control of the human tongue musculature. Exp Brain Res 173:165–173
Tachibana RO, Yanagida M, Riquimaroux H (2010) Novel approach for understanding the neural mechanisms of auditory-motor control: pitch regulation by finger force. Neurosci Lett 482:198–202
Takada T, Miyamoto T (2004) A fronto-parietal network for chewing of gum: a study on human subjects with functional magnetic resonance imaging. Neurosci Lett 360:137–140
Takeda T, Shibusawa M, Sudal O, Nakajima K, Ishigami K, Sakatani K (2010) Activity in the premotor area related to bite force control: a functional near-infrared spectroscopy study. Adv Exp Med Biol 662:479–484
Takeuchi H, Ikeda T, Clark GT (2001) A piezoelectric film-based intrasplint detection method for bruxism. J Prosthet Dent 86:195–202
Talairach J, Tournoux P (1988) Co-planar stereotaxic atlas of the human brain, George thieme verlag stuttgart. Thieme Medical Publishers Inc., New York
Tamura T, Kanayama T, Yoshida S, Kawasaki T (2003) Functional magnetic resonance imaging of human jaw movements. J Oral Rehabil 30:614–622
Teismann IK, Steinstraeter O, Stoeckigt K, Suntrup S, Wollbrink A, Pantev C, Dziewas R (2007) Functional oropharyngeal sensory disruption interferes with the cortical control of swallowing. BMC Neurosci 8:62
Toronov V, Webb A, Choi JH, Wolf M, Michalos A, Gratton E, Hueber D (2001a) Investigation of human brain hemodynamics by simultaneous near-infrared spectroscopy and functional magnetic resonance imaging. Med Phys 28:521–527
Toronov V, Webb A, Choi JH, Wolf M, Safonova L, Wolf U, Gratton E (2001b) Study of local cerebral hemodynamics by frequency- domain near-infrared spectroscopy and correlation with simultaneously acquired functional magnetic resonance imaging. Opt Express 9:417–427
van Duinen H, Zijdewind I, Hoogduin H, Maurits N (2005) Surface EMG measurements during fMRI at 3T: accurate EMG recordings after artifact correction. Neuroimage 27:240–246
Wong D, Dzemidzic M, Talavage TM, Romito LM, Byrd KE (2011) Motor control of jaw movements: an fMRI study of parafunctional clench and grind behavior. Brain Res 1383:206–217
Yan C, Ye L, Zhen J, Ke L, Gang L (2008) Neuroplasticity of edentulous patients with implant-supported full dentures. Eur J Oral Sci 116:387–393
Acknowledgments
This study was supported by a grant-in-aid for young scientists (B 21791921) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and a grant-in-aid for scientific research (C22592164 and C 23592870) from the Japanese Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iida, T., Sakayanagi, M., Svensson, P. et al. Influence of periodontal afferent inputs for human cerebral blood oxygenation during jaw movements. Exp Brain Res 216, 375–384 (2012). https://doi.org/10.1007/s00221-011-2941-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-011-2941-3