Abstract
Patients with amnesia have deficits in declarative memory but intact memory for motor and perceptual skills, which suggests that explicit memory and implicit memory are distinct. However, the evidence that implicit motor learning is intact in amnesic patients is contradictory. This study investigated implicit sequence learning in amnesic patients with Korsakoff’s syndrome (N = 20) and matched controls (N = 14), using the classical Serial Reaction Time Task and a newly developed Pattern Learning Task in which the planning and execution of the responses are more spatially demanding. Results showed that implicit motor learning occurred in both groups of participants; however, on the Pattern Learning Task, the percentage of errors did not increase in the Korsakoff group in the random test phase, which is indicative of less implicit learning. Thus, our findings show that the performance of patients with Korsakoff’s syndrome is compromised on an implicit learning task with a strong spatial response component.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple memory systems are postulated to underlie the differences in memory performance of distinct patient groups. For example, Squire (1992) distinguished between explicit or declarative memory and implicit or nondeclarative memory. The memory profile of patients with amnesic syndrome, which is characterized by deficits in declarative memory and intact memory for motor and perceptual skills, supports this distinction. (Schacter 1987; Squire and Zola-Morgan 1988). However, recent evidence suggests that implicit memory is not unconditionally spared in patients with amnesia and that in these patients, visual feedback is critical for the successful mastery of implicit movement sequences (Swinnen et al. 2005).
The participants of the present study were patients with Korsakoff’s syndrome, a disorder characterized by profound anterograde amnesia and severe, temporally graded, retrograde amnesia (Kopelman 2002). Their amnesia is a result of damage in the diencephalon, notably the mammillary bodies and thalamus (Kopelman 2002). These areas are thought to be critical for episodic memory formation, since they are crucial for storing information in the neocortex that has been integrated by the hippocampus. Mayes (1988) suggested that patients with Korsakoff’s syndrome especially have problems with memory for contextual information, such as spatial relations between stimuli, and indeed many studies have reported spatial memory deficits in these patients (Postma et al. 2006; Van Asselen et al. 2005). These studies involved explicit memory; however, evidence concerning implicit spatial memory deficits is less conclusive. While implicit spatial memory in patients with Korsakoff’s syndrome was reported to be spared in an object-location memory task (Postma et al. 2008), Chun and Phelps (1999) reported it to be impaired in a group of amnesic patients that also included Korsakoff’s patients. Because of these contradictory findings, we investigated spatial and nonspatial implicit motor learning in patients with Korsakoff’s syndrome and in healthy controls, using two implicit motor learning tasks. Motor learning refers to the increasing spatial and temporal accuracy of movements with practice (Willingham 1999). It involves more than merely gaining new movement patterns, such as in sports, and is generally defined as a process of acquiring the capability for producing skilled actions as a result of practice and which leads to relatively permanent changes in this capability (Schmidt and Wrisberg 2000). We focused on implicit sequence learning, i.e., learning the order of the submovements of an action. To this end, we used two paradigms: a standard Serial Reaction Time task (SRTT) and a newly developed, more spatially demanding, Pattern Learning task (PLT). The latter is based on the SRTT paradigm but requires the manipulation of a hand-held stylus (Van Tilborg and Hulstijn 2010).
The SRTT developed by Nissen and Bullemer (1987) is one of the most widely used tasks to study the implicit sequence learning in experimental research. In this task, participants are presented with successive visual stimuli that appear at different screen locations, to which they are asked to respond by pressing spatially corresponding keys. Initially, the stimuli are presented in a random order, but at some point in time, and unknown to the participants, they are presented in a fixed sequence. After several fixed-sequence blocks, the fixed sequence switches to a random stimulus order, to test whether sequence learning has occurred in the preceding trial blocks. An increase in reaction time after this switch reflects sequence-specific learning. Two studies in which the SRTT was administered to patients with Korsakoff’s syndrome showed that implicit learning was intact in these patients (Nissen and Bullemer 1987; Nissen et al. 1989). In contrast, a study using a different implicit motor learning task, a maze task, found implicit learning to be impaired in patients with Korsakoff’s syndrome (Nissen et al. 1989). This suggests that implicit learning may be task dependent. The SRTT is an implicit learning task in which sequences of four different finger responses are learned, resembling learning to type frequently used words. In this task, actions vary little and responses are differentiated by only four spatial locations. The SRTT shows little spatial variation regarding the nature of the response (pushing a button with one finger); the responses are four different finger movements.
Spatial aspects are suggested to be important in motor learning (Witt and Willingham 2006). Witt and Willingham stated that most types of motor skills require learning a sequence of different actions. For example, maze learning involves learning to correctly manipulate an object via a series of movements into different directions, which is comparable to learning to serve in tennis. Thus, spatial aspects play a dominant role in maze learning. Also in the PLT, where a pen is moved toward different targets, spatial aspects are more involved because the pen can be moved in three possible directions toward the target, dependent on the previous target location. In particular, the planning and execution of the motor response in the PLT have a strong spatial character. Chun and Phelps (1999) earlier reported that amnesic patients had normal implicit skills learning on nonspatial tasks, but demonstrated deficits in implicit spatial learning, which suggests that it is crucial to take the spatial aspects of tasks into account.
We investigated spatial and nonspatial implicit motor learning in patients with Korsakoff’s syndrome and in healthy controls, using two implicit learning tasks that require different types of sequential motor actions: the classic SRTT and the PLT. We expected that the patients with Korsakoff’s syndrome would show intact implicit learning on the SRTT, but compromised implicit spatial memory performance on the PLT.
Methods
Participants
Twenty patients with Korsakoff’s syndrome (16 men), inpatients of the Korsakoff Clinic of the Vincent van Gogh Institute for Psychiatry, Venray, the Netherlands, participated in this study. All met the criteria for DSM-IV Alcohol-Induced Persisting Amnestic Disorder (American Psychiatric Association 1994) and the criteria for Korsakoff’s syndrome described by Kopelman (2002). All patients had severe amnesia, measured with the Dutch version of the Rivermead Behavioral Memory Test (Wilson et al. 1985) (see Table 1), and an extensive history of alcoholism and nutritional depletion, verified by medical charts or family reports. The Mini-Mental State Examination (MMSE; Folstein et al. 1975) was administered to assess the overall cognitive functioning. Patients with a MMSE score below 17 were excluded because they might have difficulty understanding task instructions and the computer tasks. All included patients had MMSE scores higher than 20; their scores reflected the orientation and memory problems associated with their diagnosis. None of the patients fulfilled the criteria for alcohol dementia (Oslin et al. 1998). All patients underwent a comprehensive neuropsychological evaluation (see Table 1), which revealed some executive disorders, in addition to amnesic problems, but most had intact visuospatial abilities, as measured with the complex figure of Rey. The patients were matched for age and estimated general intelligence, measured with the Dutch version of the National Adult Reading Test (NART; Nelson and O'Connell 1978), with 14 healthy controls (seven men) who were either recruited from the hospital’s staff or volunteers; none had a history of neurological or psychiatric disease or subjective memory complaints. Their demographic details are given in Table 1. There were no significant differences between the control and patient groups in age (t(32) = −1.16, P = 0.255) and estimated intelligence (t(32) = 1.56, P = 0.128). All participants gave their written informed consent.
Tasks and procedure
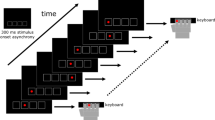
For the SRTT, the participants were seated in front of a computer monitor with a four-key response box placed directly below it. Four horizontally aligned squares, reflecting the alignment of the response keys, were always visually displayed at the bottom of the screen. In each trial, a stimulus (an asterisk) would appear in one of the four positions, but never in the same position twice in succession. The participants were instructed to press the key that corresponded to the square in which the asterisk appeared as rapidly, but also as accurately, as possible. The asterisk remained on the screen until the correct button had been pressed, after which it disappeared. The next stimulus appeared after 500 ms. The actual test comprised six trial blocks, each consisting of 100 trials. In the first block (R1), the stimuli were presented in a pseudo-random order. In the next four blocks (L1-L4), a fixed ten-trial sequence (D–B–C–A–C–B–D–C–B–A) was repeated ten times, and in the sixth block (R2), the stimuli were again presented in a pseudo-random order. The participants were not informed about the repeated sequence.
The participants sat in front of the computer to perform the Pattern Learning Task on a sheet of paper that was fixed to a digitizer (WACOM) using a normal-looking, nonink pen to control the cursor on the screen. Four circles (2.6 cm in diameter) were always visible on the screen. In each trial, one circle turned red (see Fig. 1). Participants were instructed to move the cursor (a blue dot of 0.9-cm diameter) toward the red target by means of the pen as quickly as possible. After the cursor had been inside the target for 200 ms, a beep lasting 200 ms sounded, which indicated that the next trial would start, with another circle turning red. The target remained red until the cursor had been moved inside the target. The actual test comprised six blocks of 100 trials each, with a short break (several minutes) in between the blocks. A first pseudo-random trial block (R1) was followed by four blocks (L1-L4) with a fixed sequence that was repeated ten times, after which in the sixth block (R2) another pseudo-random sequence of stimuli was presented. Again, the participants were not informed about the repeated sequence. For more methodological details on the used SRTT and the PLT, see Van Tilborg and Hulstijn (2010).
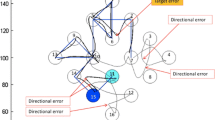
An example of a ten-trial sequence (A–D–B–C–A–C–B–D–C–B) of the Pattern Learning Task (PLT). The upper panel shows the pen trajectories, and the lower panel displays the absolute velocity of the ten trials. The upper panel depicts the four possible target locations (A, B, C and D) (visible for the participant as open black circles), as well as the cursor (real color: dark blue) and the target (real color: red) positioned at the start of the first movement (from A to D). Directional errors were made in the 5th trial (from A to C) and the 8th trial (from D to C), because these trajectories started in a direction (determined at the periphery; shown as a dotted circle only for A), which deviated by more than 22.5 degrees from the ideal direction. The display seen by the participants consisted only of the dark blue pen cursor and the four black circles (one filled red as the target) positioned in the middle of the PC screen
All participants were examined individually. They always performed the MMSE as the second and the Dutch version of the NART as the fourth component of the test session. Half of the participants started with the SRTT, the other half with the PLT. After the test session, all participants were asked whether they had noticed anything about the tasks, to establish whether they had become aware of the tasks’ fixed sequences. They could not be asked after completion of individual tests because our experimental design (two implicit sequence learning tests) necessitated the participants remaining naive with regard to the fixed sequence till the end of the test session.
Data analysis and statistical analysis
Learning in the SRTT and the PLT involves both visuomotor learning and sequence-specific learning. Accordingly, the general decrease observed across learning trials—in this study from block R1 to block L4—is interpreted as the combined result of these two learning components. However, from block L4 to block R2, the effect of task-specific sensorimotor learning will be minimal compared with the disruption caused by the change from a fixed target sequence to a random sequence. The difference between the second random block (R2) and the previous fixed-sequence block (L4) can be regarded as a measure of sequence learning (Knopman and Nissen 1987). Sequence learning is also reflected by an increase in the number of errors made when the order of the stimuli changes from fixed (in block L4) to random (in block R2). Therefore, an increase in reaction time (RT) and in the proportion of errors (pressing the wrong button) recorded in the second random block (R2) relative to the fourth fixed-sequence block (L4) were used as measures of implicit sequence learning for the SRTT. Only correct responses were included in the RT analysis.
PLT performance was recorded and analyzed by means of OASIS software (De Jong et al. 1996). In this task, the increase in total time (TT) in the last random block (R2) relative to that recorded in the last fixed-sequence block (L4) was taken as a measure of implicit learning. TT was subsequently divided into the time needed to initiate a movement (RT) and the time needed to cross the distance between the two circles (movement time or MT). RT was defined as the time between stimulus presentation and the time at which the pen left the start circle and crossed its 0.4-cm periphery (total diameter: 3.4-cm). This value was measured rather than velocity (a change from standstill to movement) because participants were allowed to start moving the pen toward the anticipated next stimulus before it was actually displayed. This instruction stimulated participants to move more or less continuously, with only very short intermittent stops, between successive target movements, but made it impossible to define reaction time based on a velocity threshold. MT was defined as the time taken to cross the distance between the periphery of the start circle and the periphery of the target circle. The TT, RT, and MT analyses excluded trials in which a directional error had been made.
Directional errors in the PLT (DE; see Fig. 1) were defined as movements that left the start circle at the wrong angle, i.e., deviations >22.5 degrees from the most optimal angle. Thus, in the PLT an error only reflects the choice of a nonoptimal movement direction in the first phase of the movement toward the target, a direction that can be corrected during the later stages of the movement. An increase in error rate in block R2 relative to that recorded for L4 was taken to indicate implicit sequence learning.
Repeated-measures multivariate tests (GLM) were conducted with block (2 levels: L4-R2) as within-subject factor and group (patients vs controls) as between-subject factor. Overall group differences were analyzed over blocks R1-L4. Furthermore, repeated-measure multivariate analyses (GLM) were conducted with block (2 levels: L4-R2) as within-subject factor and explicit knowledge (explicit knowledge vs no-explicit knowledge) as between-subject factor in both the patient and control group. Alpha was set at 0.05 throughout the study. Correlations were computed between reaction time and percentage of errors to investigate the speed-accuracy trade-off in both tasks.
Results
SRTT performance
The mean RTs for the two groups on each of the six blocks are presented in Fig. 2. As expected, the controls had significantly lower mean RTs than the patients (F(1,32) = 17.30, P < 0.001), and, also as anticipated, both groups showed implicit learning of the fixed sequence, as reflected by an increase in RT between blocks L4 and R2 (F(1,32) = 97.23, P < 0.001). The increase from L4 to R2 was not significantly different between the groups: the interaction between Group and Block was not significant (F(1,32) = 1.99, P = 0.168).
The mean SRTT error rate on the six blocks for both groups is presented in Fig. 3. The percentage of errors did not differ significantly between the groups (F(1,32) = 0.57, P = 0.46) and increased significantly between blocks L4 and R2 (F(1,32) = 10.74, P = 0.003). The Group × Block interaction was not significant (F(1,32) = 0.74, P = 0.395).
Correlations between error rates and RTs in blocks L4 and R2 within the two groups were not significant (L4: r = 0.12 and r = 0.03; R2: r = −0.07 and r = −0.16 for patients and controls, respectively).
PLT performance
Figure 4 presents the means and standard errors for the TTs, RTs, and MTs on the six blocks of the PLT for both groups.
The controls had significantly lower mean TTs than the patients (F(1,32) = 19.23, P < 0.001), and both groups showed implicit learning of the fixed sequence, as shown by the increase in TT between blocks L4 and R2 (F(1,32) = 39.08, P < 0.001); the Group × Block interaction was not significant (F(1,32) = 0.64, P = 0.430). The increase in RT between blocks L4 and R2 reflects implicit mastery of the fixed sequence in both groups (F(1,32) = 41.23, P < 0.001); the Group × Block interaction was not significant (F(1,32) = 1.12, P = 0.297). The MTs in blocks L4 and R2 (F(1,32) = 8.93, P = 0.005) also revealed a between-block difference; however, for both groups, the MTs were significantly lower in block R2, not higher. The Group × Block interaction was not significant (F(1,32) = 2.19, P = 0.148).
The mean DE rate on the six blocks for both groups is presented in Fig. 5. The DEs between blocks L4 and R2 were significantly different in the two groups, with there being a significant interaction between group and block (F(1,32) = 10.86, P = 0.002). Paired t tests (L4-R2) showed a significant increase in the control group (t(13) = −6.93, P < 0.001), but not in the Korsakoff group (t(19) = −0.71, P = 0.49).
The correlations between RT and DE rate were large and significant. In the control group, the correlation changed from r = −0.57 (P = 0.034) in block L4 to r = −0.96 (P < 0.001) in block R2. In the Korsakoff group, the correlation remained the same (r = −0.75, P < 0.001 in block L4, and r = −0.75, P < 0.001 in block R2).
Explicit knowledge
Eleven participants (five Korsakoff patients [25%] and six controls [43%]) remarked that they felt the stimuli were not administered totally at random in the SSRT, and 13 participants (seven Korsakoff patients [35%] and six controls [43%]) made the same comment regarding the PLT, implying they had some explicit knowledge of the sequences. Because of the experimental design, participants were asked about this only at the end of the study. However, in the control group the results of participants with and without knowledge were similar on the SRTT (RT: F(1,12) = 0.81, P = 0.39; error rate: F(1,12) = 1.02, P = 0.34) and the PLT (RT: F(1,12) = 1.18, P = 0.30; DE: F(1,12) = 0.43, P = 0.53), and this was also true for the Korsakoff group: SSRT (RT: F(1,18) = 0.03, P = 0.87; DE: F(1,18) < 0.01, P > 1.00) and PLT (RT: F(1,18) = 0.001, P = 0.97; DE: F(1,18) = 1.41, P = 0.25).
Discussion
In this study, we investigated the implicit learning abilities of patients with amnesia compared with healthy controls. As expected, implicit learning of the motor sequences of the SRTT was similar in the patients with Korsakoff’s syndrome and in the matched controls, as reflected by the RTs, and learning of the PLT was worse in the patients with Korsakoff’s syndrome than in the controls, as reflected by the difference in accuracy.
The RTs of all participants increased in the random trial block of the SRTT relative to the RTs in the preceding fixed trial block, which reflects implicit sequence learning in this task (Knopman and Nissen 1987). Likewise, the TTs of all participants increased in the final random block after the fixed-sequence blocks in the PLT, which reflects implicit learning. Further analysis of TT as a combination of RT and MT showed that the RT pattern corresponded with the TT pattern, while the MTs did not increase in the final random block (they even decreased). Both groups learned to perform the required movements faster over the course of all trial blocks. Implicit learning was exclusively reflected by the increase in RT in the final blocks.
We also analyzed the number of errors made in the two tasks. On the PLT, the number of directional errors (DEs) in the final random block sharply increased in the controls but not in the patients with Korsakoff’s syndrome, showing that the implicit learning of the patients was worse than that of the controls. Additional analyses of other error measures on the PLT (i.e., distance from the ideal straight line and detour of the pen trajectory) revealed similar results, showing significant group by block (L4-R2) interactions. There were no differences in error measures on the SRTT between the two groups. This difference in outcome between the two tasks can be explained by the spatial response component of the PLT. The finding that implicit spatial learning was compromised in the patients with Korsakoff’s syndrome compared to the controls is consistent with the earlier results of Chun and Phelps (1999), who reported intact implicit skill learning and impaired implicit spatial learning in amnesic patients.
Another explanation for the difference in results between the two tasks, demonstrated by the error measures, is that the frequency and type of errors made in the PLT are different from those made in the SRTT. In the PLT, an error reflects the choice of a nonoptimal movement direction in the first phase of the movement toward the target, which can be corrected during the later stages of the movement. In the SRTT, an error is the result of pressing the wrong button, which only can be corrected (and had to be corrected) by pressing another button. Because our participants were allowed to leave the start circle in the PLT before the new target circle lit up, they favored starting speed over accuracy, resulting in high DE rates during the random- and fixed-sequence blocks, and strong negative correlations between RT and error percentage. Possibly, these high DE rates might provide a more sensitive measure of implicit learning. However, this difference between the two tasks in the sensitivity of their error measures cannot explain the differences between the groups in the amount of implicit learning shown in the PLT.
The differences between the SRTT and PLT tasks regarding the magnitude of the movements made, very small discrete finger presses in the former and pen movements of 6–10 cm in the latter, and the ability to measure directional errors that could be corrected in the following movement in the PLT could underlie the differences on the tasks, rather than the difference in the spatial response component between both tasks. But this argument does not answer the question why patients with Korsakoff’s syndrome learned the PLT less well than the control group did. The PLT is somewhat similar to a maze task, and the finding of impaired learning of patients with Korsakoff’s syndrome in a maze task (Nissen et al. 1989) strengthens our argument that it is the spatial character of the planning and execution of the response in the PLT that was responsible for the poor learning of the PLT by the patients with Korsakoff’s syndrome in the present study. Moreover, group differences in speed-accuracy trade-off are unlikely to explain the differences in results between the controls and Korsakoff groups on the PLT because correlation analyses of PLT data revealed that RT and DE were significantly and substantially (negatively) correlated in both groups. Controls made more errors in the last random block than did the Korsakoff group. This indicates that the implicit learning in this task was worse in the Korsakoff group than in the control group.
The comprehensive neuropsychological evaluation of the patient group showed that most patients also had nonmemory cognitive deficits that may have interfered with their ability to learn a skill. However, the majority of patients with Korsakoff ‘s syndrome did not have visuospatial disabilities, and thus such deficits cannot explain the difference in the results on the two tasks in the patients with Korsakoff’s syndrome. Although these patients had executive deficits in the Tower of London test, which measures planning abilities, neither task required planning abilities for its performance. Mental flexibility and sensitivity to interference were intact in the majority of patients with Korsakoff’s syndromes, but their psychomotor speed was slower than that of the controls; however, all patients could complete the Trail Making Test in accordance with the test’s instruction. The overall decreased psychomotor speed of the patients with Korsakoff’s syndrome is also reflected by the significant between-group differences in the time measures in both tasks. However, this overall slowing cannot explain the difference in error measure between the two tasks.
In this study, several participants remarked that the sequences were not totally random. This indicates that they had some explicit knowledge of the sequence and thus that their test results were not the result of purely implicit learning. However, participants reported this feeling only when questioned about it: none spontaneously reported sequence knowledge. Furthermore, the remark that the sequences were not totally random after an explicit question might be the result of a positive response bias, which has been demonstrated in patients with amnesia. Our findings suggest that some knowledge of the fixed sequence did not influence implicit learning in either group of participants. Thus, limited explicit knowledge of the sequence cannot explain the difference in results on the PLT between the patient and the control groups.
As already outlined in the Introduction and in our previous study on implicit learning in patients with Parkinson’s disease or Alzheimer’s disease (Van Tilborg and Hulstijn 2010), sequential motor skills are based on multiple processes. Sequences can represent the order in which the stimuli occur, such as a visuospatial representation, or the order in which the associated movements are made, e.g., the sequence of key presses. These two types of representation may also be dissociated at a neurocognitive level. Hikosaka et al. (1999) and Nakahara Doya and Hikosaka (2001) argue that a visual loop exists that is implicated in the representation of visual coordinates and that is distinct from a motor loop, which is implicated in the representation of motor coordinates. The spatial loop comprises the association cortex (especially the prefrontal cortex) and the anterior portion of the basal ganglia, while the motor loop comprises the premotor–motor cortex (especially the SMA) and the middle portion of the basal ganglia (Hikosaka et al. 1999). It can be hypothesized that implicit learning involving the visual loop is compromised in patients with Korsakoff’s syndrome, because diencephalic regions are damaged and prefrontal regions are atrophic in these patients. This might explain why implicit learning was intact when assessed with the time measures and compromised when assessed with the number of errors on the PLT. Since in the PLT the spatial aspects are more pronounced compared to the SRTT, these tasks show different results.
In conclusion, our findings confirm the conjecture that implicit learning is compromised in patients with Korsakoff’s syndrome if the task has a strong spatial response component as in the PLT, but is spared if the task has a minimal spatial response component, as in the traditional SRTT. Thus, conclusions drawn about the extent of a patient’s implicit learning abilities need to take the type of task into account. Smith and McDowall (2006) have already argued that SRTT sequence learning is not a unitary phenomenon handled by a single general-purpose sequence learning system. We contend that the same might be true for the PLT, in which the expectation of the spatial location of the next target might be separated from learning to make the right movement toward the new target. Our findings emphasize the importance of discriminating between time and error measures in motor learning tasks.
References
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders, 4th edn. American Psychiatric Association, Washington, DC
Chun MM, Phelps EA (1999) Memory deficits for implicit contextual information in amnesic subjects with hippocampal damage. Nat Neurosci 2:844–847
De Jong WP, Hulstijn W, Kosterman BJM, Smits-Engelsman BCM (1996) OASIS software and its application in experimental handwriting research. In: Simner ML, Leedham CG, Thomassen AJWM (eds) Handwriting and drawing research: basic and applied issues. IOS Press, Amsterdam, pp 429–440
Folstein MF, Folstein SE, McHugh PR (1975) Mini-Mental State: a practical method of grading the cognitive state of patients for the clinician. J Psychiat Res 12:189–198
Hikosaka O, Nakahara H, Rand MK, Sakai K, Lu X, Nakamura K, Miyachi S, Doya K (1999) Parallel neural networks for learning sequential procedures. Trends Neurosci 22:464–471
Knopman DS, Nissen MJ (1987) Implicit learning in patients with probable Alzheimer’s disease. Neurology 37:784–788
Kopelman MD (2002) Disorders of memory. Brain 125:2152–2190
Mayes RA (1988) Human organic memory disorders. Cambridge University Press, Cambridge
Nakahara H, Doya K, Hikosaka O (2001) Parallel cortico-basal ganglia mechanisms for acquisition and execution of visuomotor sequences: a computational approach. J Cognitive Neurosci 13:626–647
Nelson HE, O`Connell A (1978) Dementia: the estimation of premorbid intelligence levels using the new adult reading test. Cortex 14:234–244
Nissen MJ, Bullemer P (1987) Attentional requirements of learning: evidence from performance measures. Cognitive Psychol 19:1–32
Nissen MJ, Willingham D, Hartman M (1989) Explicit and implicit remembering: when is learning preserved in amnesia? Neuropsychologia 27:341–352
Oslin D, Atkinson RM, Smith DM, Hendrie H (1998) Alcohol related dementia: proposed clinical criteria. Int J Geriatr Psych 13:203–212
Postma A, Van Asselen M, Keuper O, Wester AJ, Kessels RPC (2006) Spatial and temporal order memory in Korsakoff patients. J Int Neuropsychol Soc 12:327–336
Postma A, Antonides R, Wester AJ, Kessels RPC (2008) Spared unconscious influences of spatial memory in diencephalic amnesia. Exp Brain Res 190:125–133
Schacter DL (1987) Implicit memory: history and current status. J Exp Psychol Learn Mem Cogn 13:501–518
Schmidt A, Wrisberg CA (2000) Motor learning and performance, 2nd edn. Human Kinetics, Champaign
Smith JG, McDowall J (2006) The implicit sequence learning deficit in patients with Parkinson’s disease: a matter of impaired sequence integration? Neuropsychologia 44:275–288
Squire LR (1992) Declarative and nondeclarative memory: multiple brain systems supporting learning and memory. J Cogn Neurosci 4:232–243
Squire LR, Zola-Morgan S (1988) Memory: brain systems and behaviour. Trends Neurosci 102:210–221
Swinnen SP, Puttemans V, Lamotte S (2005) Procedural memory in Korsakoff’s disease under different movement feedback conditions. Behav Brain Res 159:127–133
Van Asselen M, Kessels RP, Wester AJ, Postma A (2005) Spatial working memory and contextual cueing in patients with Korsakoff amnesia. J Clin Exp Neuropsyc 27:645–655
Van Tilborg IADA, Hulstijn W (2010) Implicit motor learning in patients with Parkinson’s and Alzheimer’s disease: Differences in learning abilities? Mot Control 14:344–361
Willingham DB (1999) The neural basis of motor-skill learning. Curr Dir Psychol Sci 8:178–182
Wilson BA, Cockburn J, Baddeley A (1985) Rivermead Behavioural Memory Test (RBMT). Thames Valley Test Company, Bury St Edmunds
Witt JK, Willingham DT (2006) Evidence for separate representations for action and location in implicit motor sequencing. Psychon B Rev 13:902–907
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Van Tilborg, I.A.D.A., Kessels, R.P.C., Kruijt, P. et al. Spatial and nonspatial implicit motor learning in Korsakoff’s amnesia: evidence for selective deficits. Exp Brain Res 214, 427–435 (2011). https://doi.org/10.1007/s00221-011-2841-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-011-2841-6