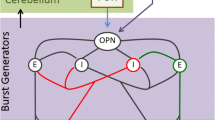
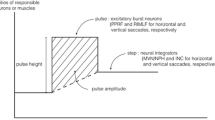
Abstract
Huntington’s disease (HD), a progressive neurological disorder involving degeneration in basal ganglia structures, leads to abnormal control of saccadic eye movements. We investigated whether saccadic impairments in HD (N = 9) correlated with clinical disease severity to determine the relationship between saccadic control and basal ganglia pathology. HD patients and age/sex-matched controls performed various eye movement tasks that required the execution or suppression of automatic or voluntary saccades. In the “immediate” saccade tasks, subjects were instructed to look either toward (pro-saccade) or away from (anti-saccade) a peripheral stimulus. In the “delayed” saccade tasks (pro-/anti-saccades; delayed memory-guided sequential saccades), subjects were instructed to wait for a central fixation point to disappear before initiating saccades towards or away from a peripheral stimulus that had appeared previously. In all tasks, mean saccadic reaction time was longer and more variable amongst the HD patients. On immediate anti-saccade trials, the occurrence of direction errors (pro-saccades initiated toward stimulus) was higher in the HD patients. In the delayed tasks, timing errors (eye movements made prior to the go signal) were also greater in the HD patients. The increased variability in saccadic reaction times and occurrence of errors (both timing and direction errors) were highly correlated with disease severity, as assessed with the Unified Huntington’s Disease Rating Scale, suggesting that saccadic impairments worsen as the disease progresses. Thus, performance on voluntary saccade paradigms provides a sensitive indicator of disease progression in HD.







Similar content being viewed by others
References
Albin RL, Reiner A, Anderson KD, Penney JB, Young AB (1990) Striatal and nigral neuron subpopulations in rigid Huntington’s disease: implications for the functional anatomy of chorea and rigidity-akinesia. Ann Neurol 27(4):357–365
Amador N, Schlag-Rey M, Schlag J (2004) Primate antisaccade II. supplementary eye field neural activity predicts correct performance. J Neurophys 91:1672–1689
Aylward EH, Sparks BF, Field KM, Yallapragada V, Shpritz BD, Rosenblatt A, Brandt J, Gourley LM, Liang K, Zhou H, Margolis RL, Ross CA (2004) Onset and rate of striatal atrophy in preclinical Huntington’s disease. Neurology 63:66–72
Berardelli A, Noth J, Thompson P, Bollen E, Curra A, Deuschl G, van Dijk JG, Töpper R, Schwarz M, Roos R (1999) Pathophysiology of chorea and bradykinesia in Huntington’s disease: review. Mov Disord 14(3):398–403
Blekher T, Johnson SA, Marshall BS, White K, Hui MS, Weaver M, Gray J, Yee R, Stout JC, Beristain X, Wojcieszek J, Foroud T (2006) Saccades in presymptomatic and early stages of Huntington disease. Neurology 67:394–399
Blekher TM, Yee RD, Kirkwood SC, Hake AM, Stout JC, Weaver MR, Foroud TM (2004) Oculomotor control in asymptomatic and recently diagnosed individuals with the genetic marker for Huntington’s disease. Vis Res 44:2729–2736
Bollen E, Reulen JPH, Den Heyer JC, Van der Kamp W, Roos RAC, Buruma OJS (1986) Horizontal and vertical saccadic eye movement abnormalities in Huntington’s chorea. J Neurol Sci 74:11–22
Chan F, Armstrong I, Pari G, Riopelle R, Munoz D (2005) Deficits in saccadic eye- movement control in Parkinson’s disease. Neuropsychologia 43(5):784–796
Condy C, Wattiez N, Rivaud-Pechoux S, Tremblay L, Gaymard B (2007) Antisaccade deficit after inactivation of the principal sulcus in monkeys. Cereb Cortex 17(1):221–229
Crossman AR, Mitchell IJ, Sambrook MA, Jackson A (1988) Chorea and myoclonus in the monkey induced by gamma-aminobutyric acid antagonism in the lentiform complex. Brain 111:1211–1233
Dias EC, Segraves MA (1999) Muscimol-induced inactivation of monkey frontal eye field: effects on visually and memory-guided saccades. J Neurophys 81(5):2191–2214
Everling S, Munoz DP (2000) Neuronal correlates for preparatory set associated with pro-saccades and antisaccades in the primate frontal eye field. J Neurosci 20:387–400
Folstein SE (1989) Huntington’s disease: a disorder of families. John Hopkins University Press, Baltimore
Gaymard B, Ploner CJ, Rivaud S, Vermersch AI, Pierrot-Deseilligny C (1998) Cortical control of saccades. Exp Brain Res 123(1–2):159–163
Goldring JE, Dorric MC, Corneil BD, Ballantyne PA, Munoz DP (1996) Combined eye-head gaze shifts to visual and auditory targets in humans. Exp Brain Res 111(1):68–78
Goodin DS, Aminoff MJ (1992) Evaluation of dementia by event-related potentials. Review. J Clin Neurophys 9(4):521–525
Guitton D, Buchtel HA, Douglas RM (1985) Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades. Exp Brain Res 58(3):455–472
Hanes DP, Wurtz RH (2001) Interaction of the frontal eye field and superior colliculus for saccade generation. J Neurophys 85(2):804–815
Hays AV, Richmond BJ, Optician LM (1982) A UNIX-based multiple process system for real-time data acquisition and control. Proc WESCON 2:1–10
Hikosaka O, Nakamura K, Nakahara H (2006) Basal ganglia orient eyes to reward. Review. J Neurophys 95(2):567–584
Hikosaka O, Takikawa Y, Kawagoe R (2000) Role of basal ganglia in the control of purposive saccadic eye movements. Phys Rev 80(3):953–978
Hikosaka O, Wurtz RH (1985a) Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in monkey superior colliculus. J Neurophys 53(1):266–291
Hikosaka O, Wurtz RH (1985b) Modification of saccadic eye movements by GABA-related substances. II. Effect of muscimol in monkey substantia nigra pars reticulata. J Neurophys 53(1):292–308
Huntington Study Group (1996) Unified Huntington’s disease rating scale: reliability and consistency. Mov Dis, 11(2):136–142
Jackson A, Crossman AR (1984) Experimental choreoathetosis produced by injection of a gamma-aminobutyric acid antagonist into the lentiform nucleus in the monkey. Neurosci Lett, 46:41–45
Johnson KA, Cunnington R, Lansek R, Bradshaw JL, Georgiou N, Chiu E (2001) Movement-related potentials in Huntington’s disease: movement preparation and execution. Exp Brain Res 138:492–499
Kassubek J, Juengling FD, Ecker D (2005) Thalamic atrophy in Huntington’s disease co-varies with cognitive performance: a morphometric MRI analysis. Cereb Cortex 15:846–853
Kimmig H, Haussmann K, Mergner T, Lucking CH (2002) What is pathological with gaze shift fragmentation in Parkinson’s disease? J Neurol 249(6):683–692
Lasker AG, Zee DS (1996) Ocular motor abnormalities in Huntington’s disease. Vis Res 37(24):3639–3645
Lasker AG, Zee DS, Hain TC, Folstein SE, Singer HS (1988) Saccades in Huntington’s disease; slowing and dysmetria. Neurology 38:427–431
Lasker AG, Zee DS, Hain TC, Folstein SE, Singer HS (1987) Saccades in Huntington’s disease; initiation defects and distractibility. Neurology 37:364–370
Lee C, Rohrer WH, Sparks DL (1988) Population coding of saccadic eye movements by neurons in the superior colliculus. Nature 332(6162):357–360
Leigh RJ, Kennard C (2004) Using saccades as a research tool in the clinical neurosciences. Brain 127(3):460–477
Leigh RJ, Newman SA, Folstein SE, Lasker AG, Jensen BA (1983) Abnormal ocular motor control in Huntington’s disease. Neurology 33:1268–1275
Leigh RJ, Zee DS (1999) The neurology of eye movements, 3rd edn. Oxford University Press, New York
LeVasseur AL, Flanagan JR, Riopelle RJ, Munoz DP (2001) Control of volitional and reflexive saccades in Tourette’s syndrome. Brain 124(10):2045–2058
Macmillan J, Quarrel O (1996) The neurobiology of Huntington’s disease. In: Harper PS (ed) Huntington’s disease. Saunders, London, pp 317–358
Miller EK, Cohen JD (2001) An integrative theory of prefrontal cortex function. Ann Rev Neurosci 24:167–202
Mitchell J, Jackson A, Sambrook MA, Crossman AR (1989) The role of the subthalamic nucleus in experimental chorea. Evidence from 2-deoxyglucose metabolic mapping and horseradish peroxidase tracing studies Brain 112:1533–1548
Munoz DP (2002). Commentary: saccadic eye movements: overview of neural circuitry. Review. Prog Brain Res 140:89–96
Munoz DP, Broughton JR, Goldring JE, Armstrong IT (1998) Age-related performance of human subjects on saccadic eye movement tasks. Exp Brain Res 121(4):391–400
Munoz DP, Everling S (2004) Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci 5(3):218–228
O’Walker F (2007) Huntington’s disease. Lancet 369:218–228
Pierrot-Deseilligny C, Rosa A, Masmoudi K, Rivaud S, Gaymard B (1991) Saccade deficits after a unilateral lesion affecting the superior colliculus. J Neurosurg Psych 54(12):1106–1109
Purdon SE, Mohr E, Ilivitsky V, Jones BDW (1994) Huntington’s disease: pathogenesis, diagnosis and treatment. Review. J Psychiatric Neurosci 19(5):359–367
Reiner A, Medina L, Veenman CL (1998) Structural and functional evolution of the basal ganglia in vertebrates. Review. Brain Res 28(3):235–285
Scudder CA, Kaneko CS, Fuchs AF (2002) The brainstem burst generator for saccadic eye movements: a modern synthesis. Exp Brain Res 142:439–462
Segraves MA, Goldberg ME (1987) Functional properties of corticotectal neurons in the monkey’s frontal eye field. J Neurophys 58(6):1387–1419
Sharp A, Ross C (1996) Neurobiology of Huntington’s disease (Review). Neurobiol Dis 3(1):3–15
Smith M, Brandt J, Shadmehr R (2000) Motor disorder in Huntington’s disease begins as a dysfunction in error feedback control. Lett Nat 403:544–549
Sommer MA, Tehovnik EJ (1999) Reversible inactivation of macaque dorsomedial frontal cortex: effects on saccades and fixations. Exp Brain Res 124(4):429–446
Storey E, Beal MF (1993) Neurochemical substrates of rigidity and chorea in Huntington’s disease. Brain 116:1201–1222
Tian JR, Zee DS, Lasker AG, Folstein SE (1991) Saccades in Huntington’s disease: Predictive tracking and interaction between release of fixation and initiation of saccades. Neurology 41:875–881
Tsai TT, Lasker A, Zee DS (1995) Visual attention in Huntington’s disease: the effect of cueing on saccade latencies and manual reaction times. Neuropsychologia 33(12):1617–1626
Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EPJ (1985) Neuropathological classification of Huntington’s disease. Neuropath Exp Neurol 44:559–577
Winograd-Gurvich CT, Georgiou-Karistianis N, Evans A, Millist L, Bradshaw JL, Churchyard A, Chiu E, White OB (2003) Hypometric primary saccades and increased variability in visually-guided saccades in Huntington’s disease. Neuropsychologia 41(12):1683–1692
Young AB, Shoulson I, Penney JB, Starosta-Rubinstein S, Gomez F, Travers H, Ramos- Arroyo MA, Snodgrass SR, Bonilla E, Moreno H et al (1986) Huntington’s disease in Venezuela: neurologic features and functional decline. Neurology 36(2):244–249
Acknowledgments
Special thanks to the Munoz Lab members for all of their editorial assistance, Don Brien for his role in preparing some of the figures for this paper, and Kim Moore for her assistance in data collection. This work was supported by a research operating grant from the Canadian Institute of Health Research to D.P.M. and G.P.
Author information
Authors and Affiliations
Corresponding author
Additional information
A. Peltsch and A. Hoffman contributed equally.
Rights and permissions
About this article
Cite this article
Peltsch, A., Hoffman, A., Armstrong, I. et al. Saccadic impairments in Huntington’s disease. Exp Brain Res 186, 457–469 (2008). https://doi.org/10.1007/s00221-007-1248-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-007-1248-x