Abstract
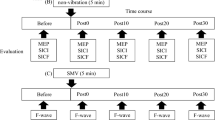
The adult human central nervous system (CNS) retains its ability to reorganize itself in response to altered afferent input. Intracortical inhibition is thought to play an important role in central motor reorganization. However, the mechanisms responsible for altered cortical sensory maps remain more elusive. The aim of the current study was to investigate changes in the intrinsic inhibitory interactions within the somatosensory system subsequent to a period of repetitive contractions. To achieve this, the dual peripheral nerve stimulation somatosensory evoked potential (SEP) ratio technique was utilized in 14 subjects. SEPs were recorded following median and ulnar nerve stimulation at the wrist (1 ms square wave pulse, 2.47 Hz, 1× motor threshold). SEP ratios were calculated for the N9, N11, N13, P14–18, N20–P25 and P22–N30 peak complexes from SEP amplitudes obtained from simultaneous median and ulnar (MU) stimulation divided by the arithmetic sum of SEPs obtained from individual stimulation of the median (M) and ulnar (U) nerves. There was a significant increase in the MU/M + U ratio for both cortical SEP components following the 20 min repetitive contraction task, i.e. the N20–P25 complex, and the P22–N30 SEP complex. These cortical ratio changes appear to be due to a reduced ability to suppress the dual input, as there was also a significant increase in the amplitude of the MU recordings for the same two cortical SEP peaks (N20–P25 and P22–N30) following the typing task. No changes were observed following a control intervention. The N20 (S1) changes may reflect the mechanism responsible for altering the boundaries of cortical sensory maps, changing the way the CNS perceives and processes information from adjacent body parts. The N30 changes may be related to the intracortical inhibitory changes shown previously with both single and paired pulse TMS. These findings may have implications for understanding the role of the cortex in the initiation of overuse injuries.






Similar content being viewed by others
References
Allison T, McCarthy G, Wood CC, Darcey TM, Spencer DD, Williamson PD (1989a) Human cortical potentials evoked by stimulation of the median nerve. II. Cytoarchitectonic areas generating short-latency activity. J Neurophysiol 62:694–710
Allison T, McCarthy G, Wood CC, Williamson PD, Spencer DD (1989b) Human cortical potentials evoked by stimulation of the median nerve. II. Cytoarchitectonic areas generating long-latency activity. J Neurophysiol 62:711–722
Allison T, McCarthy G, Wood CC, Jones SJ (1991) Potentials evoked in human and monkey cerebral cortex by stimulation of the median nerve. A review of scalp and intracranial recordings. Brain 114:2465–2503
Burke D, Gandevia SC, McKeon B, Skuse NF (1982) Interactions between cutaneous and muscle afferent projections to cerebral cortex in man. Electroencephalogr Clin Neurophysiol 53:349–360
Byl NN (2004) Focal hand dystonia may result from aberrant neuroplasticity. Adv Neurol 94:19–28
Byl NN, Melnick M (1997) The neural consequences of repetition: clinical implications of a learning hypothesis. J Hand Ther 10:160–174
Byl NN, Merzenich MM, Cheung S, Bedenbaugh P, Nagarajan SS, Jenkins WM (1997) A primate model for studying focal dystonia and repetitive strain injury: effects on the primary somatosensory cortex. Phys Ther 77:269–284
Byström SEG, Kilbom A (1990) Physiological response in the forearm during and after intermittent handgrip. Eur J Appl Physiol 60:457–466
Chen R, Corwell B, Yaseen Z, Hallett M, Cohen LG (1998) Mechanisms of cortical reorganization in lower-limb amputees. J Neurosci 18:3443–3450
Cheron G, Borenstein S (1987) Specific gating of the early somatosensory evoked potentials during active movement. Electroencephalogr Clin Neurophysiol 67:537–548
Cheron G, Borenstein S (1991) Gating of the early components of the frontal and parietal somatosensory evoked potentials in different sensory-motor interference modalities. Electroencephalogr Clin Neurophysiol 80:522–530
Cheron G, Borenstein S (1992) Mental movement simulation affects the N30 frontal component of the somatosensory evoked potential. Electroencephalogr Clin Neurophysiol 84:288–292
Classen J, Liepert J, Wise SP, Hallett M, Cohen LG (1998) Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol 79:1117–1123
Cohen LG, Starr A (1987) Localization, timing and specificity of gating of somatosensory evoked potentials during active movement in man. Brain 110:451–467
Desmedt JE, Cheron G (1980) Central somatosensory conduction in man: neural generators and interpeak latencies of the far-field components recorded from neck and right or left scalp and earlobes. Electroencephalogr Clin Neurophysiol 50:382–403
Desmedt JE, Cheron G (1981) Non-cephalic reference recording of early somatosensory potentials to finger stimulation in adult or aging normal man: differentiation of widespread N18 and contralateral N20 from the prerolandic P22 and N30 components. Electroencephalogr Clin Neurophysiol 52:553–570
Desmedt JE, Huy NT, Bourguet M (1983) The cognitive P40, N60 and P100 components of somatosensory evoked potentials and the earliest electrical signs of sensory processing in man. Electroencephalogr Clin Neurophysiol 56:272–282
Fujii M, Yamada T, Aihara M, Kokubun Y, Noguchi Y, Matsubara M, Yeh MH (1994) The effects of stimulus rates upon median, ulnar and radial nerve somatosensory evoked potentials. Electroencephalogr Clin Neurophysiol 92:518–526
Gandevia SC, Burke D, McKeon BB (1983) Convergence in the somatosensory pathway between cutaneous afferents from the index and middle fingers in man. Exp Brain Res 50:415–425
Hsieh CL, Shima F, Tobimatsu S, Sun SJ, Kato M (1995) The interaction of the somatosensory evoked potentials to simultaneous finger stimuli in the human central nervous system. A study using direct recordings. Electroencephalogr Clin Neurophysiol 96:135–142
Huttunen J, Ahlfors S, Hari R (1992) Interaction of afferent impulses in the human primary sensorimotor cortex. Electroencephalogr Clin Neurophysiol 82:176–181
Jacobs KM, Donoghue JP (1991) Reshaping the cortical motor map by unmasking latent intracortical connections. Science 251:944–947
Kanovský P, Bare M, Rektor I (2003) The selective gating of the N30 cortical component of the somatosensory evoked potentials of median nerve is different in the mesial and dorsolateral frontal cortex: evidence from intracerebral recordings. Clin Neurophysiol 114:981–991
Kim DE, Shin MJ, Lee KM, Chu K, Woo SH, Kim YR, Song EC, Lee JW, Park SH, Roh JK (2004) Musical training-induced functional reorganization of the adult brain: functional magnetic resonance imaging and transcranial magnetic stimulation study on amateur string players. Hum Brain Mapp 23:188–199
Liepert J, Classen J, Cohen LG, Hallett M (1998) Task-dependent changes of intracortical inhibition. Exp Brain Res 118:421–426
Liepert J, Kotterba S, Tegenthoff M, Malin J-P (1996) Central fatigue assessed by transcranial magnetic stimulation. Muscle Nerve 19:1429–1434
Liepert J, Terborg C, Weiller C (1999) Motor plasticity induced by synchronized thumb and foot movements. Exp Brain Res 125:435–439
Liepert J, Weiss T, Meissner W, Steinrucke K, Weiller C (2004) Exercise-induced changes of motor excitability with and without sensory block. Brain Res 1003:68–76
Mauguiere F (1999) Somatosensory evoked potentials: normal responses, abnormal waveforms and clinical applications in neurological diseases. In: Niedermeyer E (ed) Electroencephalography: basic principles, clinical applications, and related fields. Williams & Wilkins, Baltimore
Mauguiere F, Desmedt JE, Courjon J (1983) Astereognosis and dissociated loss of frontal or parietal components of somatosensory evoked potentials in hemispheric lesions. Detailed correlations with clinical signs and computerized tomographic scanning. Brain 106:271–311
Miller GA (1994) The magical number seven, plus or minus two: some limits on our capacity for processing information. Psychol Rev 101:343–352
Murphy BA, Haavik Taylor H, Wilson SA, Knight JA, Mathers KM, Schug S (2003a) Changes in median nerve somatosensory transmission and motor output following transient deafferentation of the radial nerve in humans. Clin Neurophysiol 114:1477–1488
Murphy BA, Haavik Taylor H, Wilson SA, Oliphant G, Mathers KM (2003b) Rapid reversible changes to multiple levels of the human somatosensory system following the cessation of repetitive contractions: a somatosensory evoked potential study. Clin Neurophysiol 114:1531–1537
Nuwer MR, Aminoff M, Desmedt J, Eisen AA, Goodin D, Matsuoka S, Mauguiere F, Shibasaki H, Sutherling W, Vibert JF (1994) IFCN recommended standards for short latency somatosensory evoked potentials. Report of an IFCN committee. International federation of clinical neurophysiology. Electroencephalogr Clin Neurophysiol 91:6–11
Okajima Y, Chino N, Saitoh E, Kimura A (1991) Interactions of somatosensory evoked potentials: simultaneous stimulation of two nerves. Electroencephalogr Clin Neurophysiol 80:26–31
Pedersen J, Ljubisavljevic M, Bergenheim M, Johansson H (1998) Alterations in information transmission in ensembles of primary muscle spindle afferents after muscle fatigue in heteronymous muscle. Neuroscience 84:953–959
Perneger TV (1998) What’s wrong with Bonferroni adjustments. BMJ 316:1236–1238
Pettorossi VE, Torre GD, Bortolami R, Brunetti O (1999) The role of capsaicin-sensitive muscle afferents in fatigue-induced modulation of the monosynaptic reflex in the rat. J Physiol 515:599–607
Renner CI, Schubert M, Hummelsheim H (2005) Selective effect of repetitive hand movements on intracortical excitability. Muscle Nerve 31:314–320
Ridding MC, Rothwell JC (1999) Afferent input and cortical organisation: a study with magnetic stimulation. Exp Brain Res 126:536–544
Rossi S, Tecchio F, Pasqualetti P, Ulivelli M, Pizzella V, Romani GL, Passero S, Battistini N, Rossini PM (2002) Somatosensory processing during movment observation in humans. Clin Neurophysiol 113:16–24
Rossi S, della Volpe R, Ginanneschi F, Ulivelli M, Bartalini S, Spidalieri R, Rossi A (2003) Early somatosensory processing during tonic muscle pain in humans: relation to loss of proprioception and motor ‘defensive’ strategies. Clin Neurophysiol 114:1351–1358
Rossini PM, Gigli GL, Marciani MG, Zarola F, Caramia M (1987) Non-invasive evaluation of input–output characteristics of sensorimotor cerebral areas in healthy humans. Electroencephalogr Clin Neurophysiol 68:88–100
Rossini PM, Babiloni F, Bernardi G, Cecchi L, Johnson PB, Malentacca A, Stanzione P, Urbano A (1989) Abnormalities of short-latency somatosensory evoked potentials in parkinsonian patients. Electroencephalogr Clin Neurophysiol 74:277–289
Rossini PM, Caramia D, Bassetti MA, Pasqualetti P, Tecchio F, Bernardi G (1996) Somatosensory evoked potentials during the ideation and execution of individual finger movements. Muscle Nerve 19:191–202
Schubert M, Kretzschmar E, Waldmann G, Hummelsheim H (2004) Influence of repetitive hand movements on intracortical inhibition. Muscle Nerve 29:804–811
Sonoo M, Kobayashi M, Genba-Shimizu K, Mannen T, Shimizu T (1996) Detailed analysis of the latencies of median nerve somatosensory evoked potential components, one: selection of the best standard parameters and the establishment of normal values. Electroencephalogr Clin Neurophysiol 100:319–331
Tapia MC, Cohen LG, Starr A (1987) Selectivity of attenuation (i.e., gating) of somatosensory potentials during voluntary movement in humans. Electroencephalogr Clin Neurophysiol 68:226–230
Tinazzi M, Zanette G, Polo A, Volpato D, Manganotti P, Bonato C, Testoni R, Fiaschi A (1997) Transient deafferentation in humans induces rapid modulation of primary sensory cortex not associated with subcortical changes: a somatosensory evoked potential study. Neurosci Lett 223:21–24
Tinazzi M, Zanette G, Volpato D, Testoni R, Bonato C, Manganotti P, Miniussi C, Fiaschi A (1998) Neurophysiological evidence of neuroplasticity at multiple levels of the somatosensory system in patients with carpal tunnel syndrome. Brain 121:1785–1794
Tinazzi M, Priori A, Bertolasi L, Frasson E, Mauguiere F, Fiaschi A (2000) Abnormal central integration of a dual somatosensory input in dystonia. Evidence for sensory overflow. Brain 123:42–50
Ulas UH, Odabasi Z, Ozdag F, Eroglu E, Vural O (1999) Median nerve somatosensory evoked potentials:recording with cephalic and noncephalic references. Electromyogr Clin Neurophysiol 39:473–477
Waberski TD, Buchner H, Perkuhn M, Gobbele R, Wagner M, Kucker W, Silny J (1999) N30 and the effect of explorative finger movements: a model of the contribution of the motor cortex to early somatosensory potentials. Clin Neurophysiol 110:1589–1600
Zanette G, Bonato C, Polo A, Tinazzi M, Manganotti P, Fiaschi A (1995) Long-lasting depression of motor-evoked potentials to transcranial magnetic stimulation following exercise. Exp Brain Res 107:80–86
Ziemus B, Huonker R, Haueisen J, Liepert J, Spengler F, Weiller C (2000) Effects of passive tactile co-activation on median ulnar nerve representation in human SI. Neuroreport 11:1285–1288
Acknowledgments
Heidi Haavik-Taylor was supported by a New Zealand Tertiary Education Commission Top Achiever Doctoral Scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haavik Taylor, H., Murphy, B.A. Altered cortical integration of dual somatosensory input following the cessation of a 20 min period of repetitive muscle activity. Exp Brain Res 178, 488–498 (2007). https://doi.org/10.1007/s00221-006-0755-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-006-0755-5