Abstract
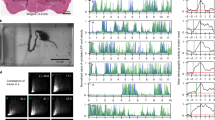
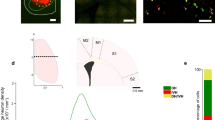
The dorsal spinocerebellar tract (DSCT) provides a major mossy fiber input to the spinocerebellum, which plays a significant role in the control of posture and locomotion. Recent work from our laboratory has provided evidence that DSCT neurons encode a global representation of hindlimb mechanics during passive limb movements. The framework that most successfully accounts for passive DSCT behavior is kinematics-based having the coordinates of the limb axis, limb-axis length and orientation. Here we examined the responses of DSCT neurons in decerebrate cats as they walked on a moving treadmill and compared them with the responses passive step-like movements of the hindlimb produced manually. We found that DSCT responses to active locomotion were quantitatively different from the responses to kinematically similar passive limb movements on the treadmill. The differences could not be simply accounted for by the difference in limb-axis kinematics in the two conditions, nor could they be accounted for by new or different response components. Instead, differences could be attributed to an increased relative prominence of specific response components occurring during the stance phase of active stepping, which may reflect a difference in the behavior of the sensory receptors and/or of the DSCT circuitry during active stepping. We propose from these results that DSCT neurons encode two global aspects of limb mechanics that are also important in controlling locomotion at the spinal level, namely the orientation angle of the limb axis and limb loading. Although limb-axis length seemed to be an independent predictor of DSCT activity during passive limb movements, we argue that it is not independent of limb loading, which is likely to be proportional to limb length under passive conditions.






Similar content being viewed by others
References
Armstrong DM, Apps R, Marple-Horvat DE (1997) Aspects of cerebellar function in relation to locomotor movements. Prog Brain Res 114:401–421
Arshavskii YI, Berkinblit MB, Gel’Fand IM, Orlovskii GN, Fukson OI (1972a) Activity of the neurones of the dorsal spino-cerebellar tract during locomotion. Biofizika 17:487–494References Arshavsky et al. 1972a has been changed to Arshavsky et al. 1972 and Arshavskii et al. 1972b, c has been changed to Arshavskii et al. 1972a, b. The following changes have been made both in the text and in the reference list. Author please check
Arshavskii YI, Berkinblit MB, Gel’fand IM, Orlovskii GN, Fukson OI (1972b) Activity of the neurones of the ventral spino-cerebellar tract during locomotion of cats with deafferentated hind limbs. Biofizika 17:1112–1118
Arshavsky YI, Berkinblit MB, Fukson OI, Gelfand IM, Orlovsky GN (1972) Recordings of neurones of the dorsal spinocerebellar tract during evoked locomotion. Brain Res 43:272–275
Arshavsky YI, Deliagina TG, Orlovsky GN (1997) Pattern generation. Curr Opin Neurobiol 7:781–789
Bennett DJ, De Serres SJ, Stein RB (1996) Regulation of soleus muscle spindle sensitivity in decerebrate and spinal cats during postural and locomotor activities. J Physiol. 495:835–850
Borghese NA, Bianchi L, Lacquaniti F (1996) Kinematic determinants of human locomotion. J Physiol 494:863–879
Bosco G, Poppele RE (2000) Reference frames for spinal proprioception: kinematics based or kinetics based? J Neurophysiol 83:2946–2955
Bosco G, Poppele RE (2001) Proprioception from a spinocerebellar perspective. Physiol Rev 81:539–568
Bosco G, Poppele R (2003a) Cerebellar afferent systems: can they help us understand cerebellar function? Cerebellum 2:162–164
Bosco G, Poppele RE (2003b) Modulation of dorsal spinocerebellar responses to limb movement II: effect of sensory input. J Neurophysiol 90:3372–3383
Bosco G, Rankin A, Poppele RE (1996) A representation of passive hindlimb postures in cat spinocerebellar activity. J Neurophysiol 76:715–726
Bosco G, Poppele RE, Eian J (2000) Reference frames for spinal proprioception: limb endpoint based or joint-level based? J Neurophysiol 83:2931–2945
Bosco G, Rankin A, Poppele RE (2003) Modulation of dorsal spinocerebellar responses to limb movement. I. Effect of serotonin. J Neurophysiol 90:3361–3371
Bosco G, Eian J, Poppele RE (2005) Kinematic and non-kinematic signals transmitted to the cat cerebellum during passive treadmill stepping. Exp Brain Res 167:394–403
Dietz V (2002) Proprioception and locomotor disorders. Nat Rev Neurosci 3:781–790
Dietz V (2003) Spinal cord pattern generators for locomotion. Clin Neurophysiol 114:1379–1389
Duysens J, Clarac F, Cruse H (2000) Load-regulating mechanisms in gait and posture: comparative aspects. Physiol Rev 80:83–133
Grillner S (2002) The spinal locomotor CPG: a target after spinal cord injury. Prog Brain Res 137:97–108
Hiebert GW, Pearson KG (1999) Contribution of sensory feedback to the generation of extensor activity during walking in the decerebrate cat. J Neurophysiol 81:758–770
Hiebert GW, Whelan PJ, Prochazka A, Pearson KG (1996) Contribution of hind limb flexor muscle afferents to the timing of phase transitions in the cat step cycle. J Neurophysiol 75:1126–1137
Ivanenko YP, Grasso R, Macellari V, Lacquaniti F (1999) Control of foot trajectory in human locomotion: role of ground contact forces in simulated reduced gravity. J Neurophysiol 87:3070–3089
Jankowska E, Krutki P, Matsuyama K (2005a) Relative contribution of la inhibitory interneurones to inhibition of feline contralateral motoneurones evoked via commissural interneurones. J Physiol 586(Pt 2):617–628
Jankowska E, Edgley SA, Krutki P, Hammar I (2005b) Functional differentiation and organization of feline midlumbar commissural interneurones. J Physiol 565(Pt 2):645–658
Lacquaniti F, Grasso R, Zago M (1999) Motor patterns in walking. News Physiol Sci 14:168–174
Lam T, Pearson KG (2001) Proprioceptive modulation of hip flexor activity during the swing phase of locomotion in decerebrate cats. J Neurophysiol 86:1321–1332
Lomeli J, Quevedo J, Linares P, Rudomin P (1998) Local control of information flow in segmental and ascending collaterals of single afferents. Nature 395:600–604
MacPherson JM (1988) Strategies that simplify the control of quadrupedal stance. I. Forces at the ground. J Neurophysiol 60:204–217
Mann MD (1973) Clarke’s column and the dorsal spinocerebellar tract: a review. Brain Behav Evol 7:34–83
Matsushita M, Hosoya Y, Ikeda M (1979) Anatomical organization of the spinocerebellar system in the cat, as studied by retrograde transport of horseradish peroxidase. J Comp Neurol 184:81–106
McCrea DA (2001) Spinal circuitry of sensorimotor control of locomotion. J Physiol 533(1):41–50
Miall RC, Wolpert DM (1996) Forward models for physiological motor control. Neural Netw 9:1265–1279
Mori S, Shik ML, Yagodnitsyn AS (1977) Role of pontine tegmentum for locomotor control in mesencephalic cat. J Neurophysiol 40:284–295
Mori S, Matsui T, Kuze B, Asanome M, Nakajima K, Matsuyama K (1998) Cerebellar-induced locomotion: reticulospinal control of spinal rhythm generating mechanism in cats. Ann N Y Acad Sci 860:94–105
Mori S, Matsui T, Kuze B, Asanome M, Nakajima K, Matsuyama K (1999) Stimulation of a restricted region in the midline cerebellar white matter evokes coordinated quadrupedal locomotion in the decerebrate cat. J Neurophysiol 82:290–300
Mori S, Nakajima K, Mori F, Matsuyama K (2004) Integration of multiple motor segments for the elaboration of locomotion: role of the fastigial nucleus of the cerebellum. Prog Brain Res 143:341–351
Morton SM, Bastianu AJ (2004) Cerebellar control of balance and locomotion. Neuroscientist 10:247–259
Murphy PR, Stein RB, Taylor J (1984) Phasic and tonic modulation of impulse rates in gamma-motoneurons during locomotion in premammillary cats. J Neurophysiol 52:228–243
Osborn CE, Poppele RE (1983) Cross-correlation analysis of the response of units of the dorsal spinocerebellar tract (DSCT) to muscle stretch and contraction. Brain Res 280:339-342
Osborn CE, Poppele RE (1992) Parallel distributed network characteristics of the DSCT. J Neurophysiol 68:1100–1112
Pearson KG (1995) Proprioceptive regulation of locomotion. Curr Opin Neurobiol 5:786–791
Pearson KG (2004) Generating the walking gait: role of sensory feedback. Prog Brain Res 143:123–129
Poppele RE, Bosco G, Rankin AM (2002) Independent representations of limb-axis length and orientation in spinocerebellar response components. J Neurophysiol 87:409–422
Poppele RE, Rankin A, Eian J (2003) Dorsal spinocerebellar tract neurons respond to contralateral limb stepping. Exp Brain Res 149:361–370
Prochazka A, Hulliger M (1998) The continuing debate about CNS control of proprioception. J Physiol 1:513
Prochazka A, Gillard D, Bennett DJ (1997) Positive force feedback control of muscles. J Neurophysiol 77:3226–3236
Rossignol S, Dubuc R (1994) Spinal pattern generation. Curr Opin Neurobiol 4:894–902
Rudomin P (1999) Presynaptic selection of afferent inflow in the spinal cord. J Physiol 93:329–347
Sillar KT (1991) Spinal pattern generation and sensory gating mechanisms. Curr Opin Neurobiol 1:583–589
Whelan PJ, Pearson KG (1997) Plasticity in reflex pathways controlling stepping in the cat. J Neurophysiol 78:1643–1650
Whelan PJ, Hiebert GW, Pearson KG (1995) Stimulation of the group I extensor afferents prolongs the stance phase in walking cats. Exp Brain Res 103:20–30
Wilkinson L (1990) The system for statistics. Evanston, IL, SYSTAT, Inc
Acknowledgements
This research was supported by a grant from the USPHS, NIH grant R01 NS21143. The authors thank Dr. M. S. Valle for help and assistance on this project, and Dr. J. Soechting for a critical reading of the manuscript and helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bosco, G., Eian, J. & Poppele, R.E. Phase-specific sensory representations in spinocerebellar activity during stepping: evidence for a hybrid kinematic/kinetic framework. Exp Brain Res 175, 83–96 (2006). https://doi.org/10.1007/s00221-006-0530-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-006-0530-7